Abstract
Hypercholesterolemia (HC) is a common health problem that significantly increases risk of cardiovascular disease. Both statin (S) and resveratrol (R) demonstrated cardioprotection through nitric oxide dependent mechanism. Therefore the present study was undertaken to determine whether combination therapy with statin and resveratrol are more cardioprotective than individual treatment groups in ischemic rat heart model. The rats were fed rats with 2% high cholesterol diet and after 8 weeks of high cholesterol diet the animals were treated with statin (1mg/kg bw/day) and resveratrol (20mg/kg bw/day) for 2 weeks. The rats were assigned to: 1) Control (C) 2) HC 3) HCR 4) HCS and 5) HCRS. The hearts, subjected to 30 min global ischemia followed by 120 min reperfusion were used as experimental model. The left ventricular functional recovery (+dp/dt) was found to be significantly better in the HCRS (1926±43), HCR (1556±65) and HCS (1635±40) compared to HC group (1127±16). The infarct size in the HCRS, HCS and HCR groups were 37±3.6, 43±3.3 and 44±4.2 respectively compared to 53±4.6 in HC. The lipid level was found to be decreased in all the treatment groups when compared to HC more significantly in HCS and HCRS groups when compared to HCR. Increased phosphorylation of Akt and eNOS was also observed in all the treatment groups resulting in decreased extent of cardiomyocyte apoptosis but the extent of reduction in apoptosis was more significant in HCRS group compared to all other groups. In-vivo rat myocardial infarction (MI) model subjected to one week of permanent left descending coronary artery (LAD) occlusion documented increased capillary density in HCR and HCRS treated group when compared to HCS treatment group. We also documented increased β-catenin translocation and increased VEGF mRNA expression in all treatment groups. Thus, we conclude that the acute as well as chronic protection afforded by combination treatment with statin and resveratrol may be due to pro-angiogenic, anti-hyperlipidemic and anti-apoptotic effects and long term effects may be caused by increased neovascularization of the MI zone leading to less ventricular remodeling.
Keywords: statin, resveratrol, cholesterol, myocardium, Akt, eNOS, VEGF, β-catenin
Introduction
Elevated levels of total cholesterol (hypercholesterolemia) and low-density lipoprotein cholesterol (LDL-C) are well-recognized risk factors of coronary heart disease (CHD) as they have been implicated in the progression of plaque formation, endothelial dysfunction and oxidative stress in CHD [1,2]. Promotion of functional collateral formation in such CHD patients is an exciting therapeutic strategy, which is popularly called as therapeutic angiogenesis, to minimize cell death associated with severe ischemia.
Recent evidences show the benefits of reducing LDL-C and cholesterol is associated with a reduced risk of CHD [3]. HMG-CoA inhibitors (Statins) therapy has been shown as the most effective means of reducing cholesterol levels thereby inhibiting the progression of atherosclerosis and reducing the incidence of CHD. Besides the cholesterol-lowering effect, statins have been shown to improve the endothelial relaxation, an antihypertensive effect and beneficial actions on cardiac fuction [4)]. Statins were found to protect the ischemic-reperfused myocardium in normocholesterolemic rats via an endothelial nitric oxide synthase (eNOS)-dependent manner [5]. However, monotherapy with statins in patients with hypercholesterolemia have shown fewer efficacies concerning the recurrences of CHD. Thus the “Holy grails of current research” include the design of rational integrated treatment regimens that act by multiple mechanisms to lower cholesterol, and reduce the CHD. In this context, we worked on a combination therapy of statin with resveratrol, which is a polyphenolic compound, and naturally occurring phytoalexin with many biological activities including anti-oxidant, anti-platelet, angiogenic and anti-inflammatory function [6]. The beneficial effect of resveratrol on coronary artery disease may be due to its ability to retard the progression of early atherosclerotic lesions. Our previous reports demonstrated significant reduction in oxidative stress and upregulation of inducible nitric oxide synthase (iNOS) mRNA expression leading to the reduction of cardiomyocyte apoptosis by resveratrol [7]. Recent report from our laboratory has also documented the cardioprotective effect of resveratrol in vivo as well as in vitro which operates through the increased induction of thioredoxin (Trx-1), heme oxygenase (HO-1) and vascular endothelial growth factor (VEGF). Moreover, we have recently demonstrated that β-catenin, a critical mediator during development and angiogenesis, is translocated into the nucleus by the phosphorylation of GSK-3β through activation of Akt and thus decrease the apoptotic cardiomyocytes by increasing the expression of VEGF, Bcl-2 and survivin expression in in vivo rat MI model which is mediated through phosphorylation of Akt [8]. Therefore in this study we investigated the effects of hypercholesterolemia on the functional and angiogenic parameters in ischemic/reperfused (HC) myocardium. We have also designed our study to examine the effects of statin, resveratrol & resveratrol and statin in combination on ischemic reperfused myocardium in the HC rats. Our results documented significant improvement in lipid profile, cardiac functions, decreased infarct size and cardiomyocyte apoptosis in a rat ischemic reperfused model along with increased angiogenesis and significant collateral vessel formation in a rat MI model under combination therapy. Hence this study provides a rationale for the combination therapy using statin and resveratrol in acquiring acute and chronic cardioprotection after myocardial infarction which is not possible with monotherapy.
Materials and Methods
Animals
All animals used in the study received human care in compliance with the principles of laboratory animal care formulated by the National Society for Medical Research and guide for the care and use of Laboratory Animals published by NIH.
Experimental Design
In the present study male Sprague-Dawley rats were fed with 2% cholesterol diet (CD) for 8 weeks. After CD diet animals were treated with resveratrol (Sigma) (20mg/kg bw/day) and pravastatin (which was a generous gift from Sankyo Pharmaceutical Co.) (1mg/kg bw/day) for 2 weeks orally. The rats were randomized into five groups. 1) Control (C) 2) Hypercholesterolemia (HC) 3) Hypercholesterolemia + Resveratrol (HCR) 4) Hypercholesterolemia + Statin (HCS) 5) Hypercholesterolemia + Resveratrol + Statin (HCRS). The Langendorff’s working perfused rat hearts, subjected to 30min of global ischemia followed by 120min reperfusion and the in-vivo myocardial infarction model subjected to 7 days of permanent LAD occlusion were used.
Determination of Lipid levels
Blood was collected from the jugular vein and transferred into heparinized tubes and the plasma is separated after centrifugation. The cholesterol, triglycerides, HDL-C levels were measured quantitatively by using kits for total cholesterol, triglycerides and HDL-C (Wako Diagnostics, Richmond, VA 23237, USA) according to the kit protocol. LDL-C was measured by using Friedewald Equation [LDL-chol] = [Total chol] - [HDL-chol] - ([TG]/5)).
Isolated Working heart
The rats were first anesthetized with sodium pentobarbital (80mg/kg bw i.p.) and anticoagulated with Heparin (500 U/kg bw i.p.). The heart was excised and immediately immersed in ice-cold perfusion buffer. The aorta was cannulated and the heart was perfused with Krebs-Henseleit Bicarbonate (KHB) buffer in the Langendorff mode through the aortic cannula at a perfusion pressure of 65 mm Hg. After 10 minutes of retrograde perfusion, the heart was switched to antegrade perfusion mode during which KHB buffer enters through the cannulated left atrium at a pressure equivalent to 10 cm of water, and passes to the left ventricle from which it was spontaneously ejected through the aortic cannula against a pressure equivalent to 100 cm of water. Control measurements of heart rate, coronary flow, aortic flow, left ventricular developed pressure (LVDP), and derivative of pressure/derivative of time (dp/dt) were recorded before ischemia and during 2 hours of reperfusion at time intervals of 30,60,90 &120 min of reperfusion [9]. Aortic pressure was measured using a pressure transducer (Micro-Med, Inc., USA) connected to a side arm of the aortic cannula, the signal was amplified using a Heart Performance Analyzer Model 400 (Micro-Med, Inc, USA). Heart rate (HR), Left ventricular developed pressure (LVDP) (defined as the difference of the maximum systolic and diastolic aortic pressures), and the first derivative of developed pressure (dP/dtmax) were all derived or calculated from the continuously obtained pressure signal. Aortic flow (AF) was measured using a calibrated flow meter (Gilmont Instrument Inc., Barrington, IL, USA) and coronary flow (CF) was measured by timed collection of the coronary effluent dripping from the heart.
Infarct size
At the end of reperfusion, 1% (W/V) solution of triphenyl tetrazolium chloride in phosphate buffer was infused into aortic cannula for 20 min at 37°C. The hearts were excised and stored at −70°C. Sections of frozen heart were fixed in 4% para-formaldehyde, placed between two glass slides and digitally imaged using Epson Scanner. To quantitate the areas of infarct in pixels, NIH image software was used. The infarct size was quantified and expressed in pixels [10].
Cardiomyocyte cell apoptosis
The rat hearts were harvested at pre-determined times as per the protocol for paraffin-embedded or frozen tissue sectioning. Double-fluorescent immunohistochemical determination of cardiomyocyte apoptosis was performed with Terminal dUTP Nick End Labeling (TUNEL) assay with deparafinized sections of 4uM thick with an Apop Tag Kit (Oncor Inc) according to kit protocol [10] using an antibody against α-sarcomeric actin (sigma). The number of TUNEL-positive cardiomyocytes was counted on 100 high power fields.
Western Blot Analysis for phosphorylated eNOS & AKT
To quantify the abundance of the p-eNOS and p-AKT, we performed standard SDS/PAGE Western blot technique. Heart tissues from each treatment group were homogenized and suspended (50 mg/ml) in sample buffer [10 mM HEPES, pH 7.3, sucrose 11.5%, EDTA 1mM, EGTA 1mM, diisopropylfluorophosphate (DFP), pepstatin A 0.7 mg/ml, leupeptin 10 mg/ml, aprotinin 2 mg/ml]. The homogenates were centrifuged at 3,000 rpm and the cytosolic fractions were used for protein analysis. The total protein concentration was determined using BCA (bicinchoninic acid) protein assay kit (Pierce, Rockville, IL). The cytosolic proteins were run on polyacrylamide electrophoretic gels (SDS-PAGE) typically using 7% & 10% (acrylamide to bis ratios). The separated proteins were electrophoretically transferred to Immobilon-P membranes (Millipore Corp., Bedford, MA) using a semidry transfer system (Bio-Rad, Hercules, CA). Protein standards (Bio-Rad) were run in each gel. The blots were blocked in Tris-buffered saline/Tween-20 (TBS-T containing 20 mM Tris base, pH 7.6, 137 mM NaCl, 0.1% Tween –20) supplemented with 5%(wt/vol) non-fat dry milk for 1 hour; blots were incubated overnight at 4°C with the various primary antibodies. The antibodies were purchased from Cell signaling laboratories and were used at manufacturer-recommended dilutions. Membranes were washed three times in TBS-T before incubation for 1h with horseradish peroxidase-conjugated secondary antibody diluted 1:2000 in TBS-T and 5% (wt/vol) nonfat dry milk. Following incubation membranes were washed three times with TBS-T for 10 min each, blots were treated with Enhanced Chemi-Luminescence (ECL from Amersham) reagents and the required proteins were detected by autoradiography for variable lengths of time with Kodak X-Omat film. [11].
Surgical procedure
Male Sprague Dawley rats weighing 450–500g were anesthetized with ketamine HCl (100mg/kg bw i.p.) and xylazine (10 mg/kg bw i.p.). Cefazolin (25 mg/kg bw i.p.) was administered as preoperative antibiotic cover. After tracheotomy and initiation of ventilation (Harvard Apparatus Rodent ventilator:Model 683), the heart was exposed through a left lateral thoracotomy (4th intercostal space). A 6–0 polypropylene suture was passed with tapered needle under the left anterior descending coronary artery (LAD) just below the tip of the left auricle, and a non-traumatic occluder was applied on the artery. Myocardial infarction was produced by permanent LAD occlusion. After completion of all surgical protocols, the chest wall was closed. After application of buprenorphine (0.1 mg/kg s.c) and weaning from the respirator, the rats were placed on a heating pad while recovering from anesthesia.
Immunohistochemistry for β-catenin translocation and Capillary density
The paraffin-embedded sections were used to clarify localization of β-catenin. Myocardial sections were analysed for the reactivity with 1:500 diluted monoclonal antibody against β-catenin (BD Transduction Labs, Lexington, KY). Bound antibody was detected with the Vectastain ABC kit (Vector Laboratories, Burlingame CA) and visualized with DAB (Sigma). Following seven days of the surgical procedure as mentioned above, the rats were sacrificed and the hearts were removed and paraffin-embedded sections were made to study capillary density (CD-31). The sections were stained for capillary density using PECAM-1 primary antibody (Santa cruz) and bound antibody was detected with Vectastain ABC kit (Vector Laboratories, Burlingame CA) and visualized with DAB (Sigma) [12]. At 400X magnification pictures were captured and used for CD-31 counting. For the quantitative measurement, the number of CD-31 was counted on area at risk from the endocardium through the epicardium of the mid portion of the left ventricular free wall. Counts of capillary density per mm2 were obtained after superimposing a calibrated morphometric grid on each digital image using Adobe Photoshop Software.
Quantitative real-time RT-PCR
Total RNA was isolated from left ventricular tissue of the rats subjected to ischemia-reperfusion using Qiagen Kit according to the kit protocol. Real-time-RT-PCR was performed on 1μg total RNA by using the iCycler iQ detection system (Biorad, Hercules, CA) by Syber Green I using β-actin as reference control. The primer used in quantitative real-time RT-PCR for VEGF was (forward,5′-TGTGCGGGCTGCTGCAATGAT-3′; reverse, 5′-TGTGCTGGCTTTGGTGAGGTTTGA-3′ [12].
Results
Effect of resveratrol and statin on lipid levels
The levels of cholesterol, triglycerides, LDL-C were found to be increased and HDL-C level was found to be decreased in HC group when compared to control. Treatment with resveratrol significantly lowered the cholesterol, triglycerides, LDL-C levels when compared to HC group. The lipid lowering ability was more prominent in HCS and HCRS (Table-1) than in HCR group.
Effect of resveratrol and statin on cardiac functions
There was no significant difference at baseline level in cardiac functions (LVDP (mmHg), heart rate (beat/min), dp/dt (mm Hg/sec), coronary flow (ml/min) and aortic flow (ml/min)) in treatment groups when compared with HC and control hearts. Following ischemia, upon reperfusion, cardiac function was found to be more significant in all the treatment groups when compared to HC group. Thus significant increase in dp/dtmax and aortic flow were observed during reperfusion in resveratrol, statin and combination groups when compared to HC group. Following 120 min of reperfusion, post ischemic values of dp/dtmax were significantly increased in all treatment groups when compared to HC group (1127.83±16.10). [HCRS (1926.83±43.24), HCR group (1556.66±65.47), HCS group (1635.66±40.36)]. Similarly, aortic flow significantly increased HCR, HCS and in HCRS groups when compared to HC group. In spite of significant functional recovery in HCR and HCS groups, the HCRS group was found to demonstrate more functional recovery when compared to individual treatment groups (monotherapy). Therefore as expected resveratrol and statin showed significant recovery of postischemic myocardial function (Figure 1A–1E) as compared to HC. However with careful observation in this case combination therapy looks more promising compared to the individual treatment groups which in our view is quite reasonable.
Figure 1.
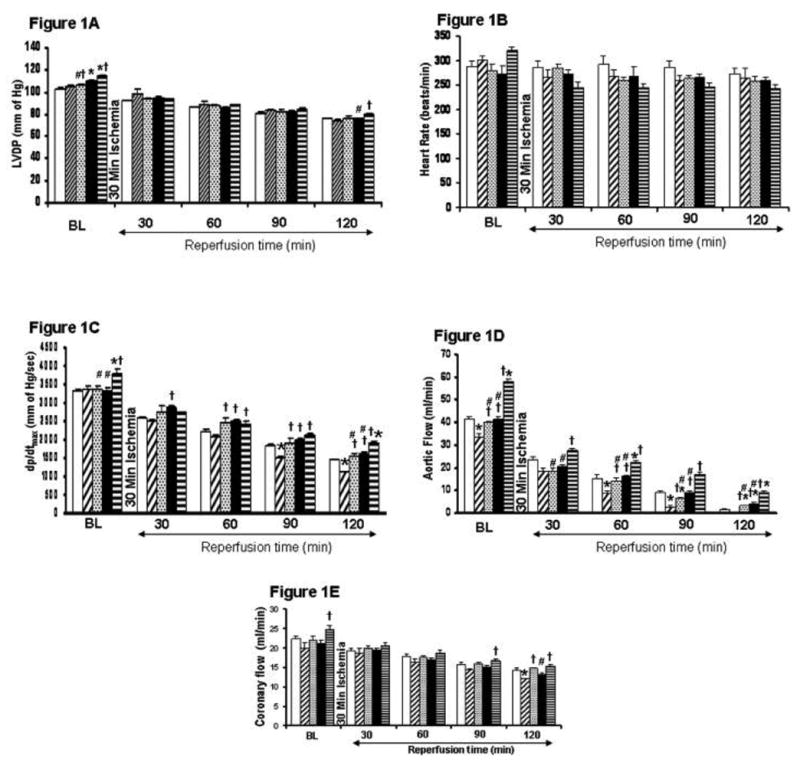
A–1E. Post ischemic ventricular recovery of resveratrol and statin treatment in hypercholesterol rats compared to control. The results (LVDP-1A, heart rate-1B, +dp/dt-1C, aortic flow-1D, coronary flow-1E) are shown in six animals per group. *P<0.05 compared to control, †P<0.05 compared to hypercholesterol group, #P<0.05 compared to HCRS group. Blank bar represents control group. Dark upward diagonal bars represent Hypercholesterol group. Dashed horizontal bars represent resveratrol treated group. Black bar represents statin treated group. Dark horizontal lines represent combination group.
Effect of resveratrol and statin on infarct size
Hearts subjected to 30 min of global ischemia followed by a 2 h reperfusion has shown significant increase in infarct size (percent of infarct vs total area at risk) in the HC group when compared to resveratrol, statin and combination groups. The values were significantly reduced in HCRS, HCS and HCR treatment (37±3.6, 43±3.3 and 44±4.2) as compared to the HC group (53.74±4.6) (Figure-2). To be more accurate combination group was found to be more effective (37±3.6) in reducing infarct when compared to statin (43±3.3) and resveratrol (44±4.2) group individually.
Figure 2.
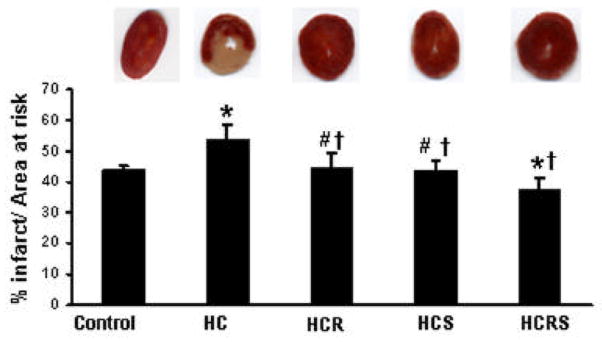
Infarct size of the hearts, expressed as a percentage of the area at risk in rat myocardium subjected to 30 min of ischemia followed by 2 hours of reperfusion. Results are expressed as mean ± Std. error of six hearts per group. *P<0.05 compared with control, †P<0.05 compared with HC group, #P<0.05 compared with HCRS group.
Effect of resveratrol and statin on extent of cardiomyocyte apoptosis
There was a significant decrease in the extent of cardiomyocyte apoptosis in HCR, HCS and HCRS groups when compared to HC group (Figure 3A and 3B). The decrease was more significant in the combination group as expected when compared to individual resveratrol and statin treatment. Reduced apoptosis in resveratrol and statin groups might be due to the activation of survival pathway as evidenced by the extent of phosphorylation of Akt and eNOS.
Figure 3.
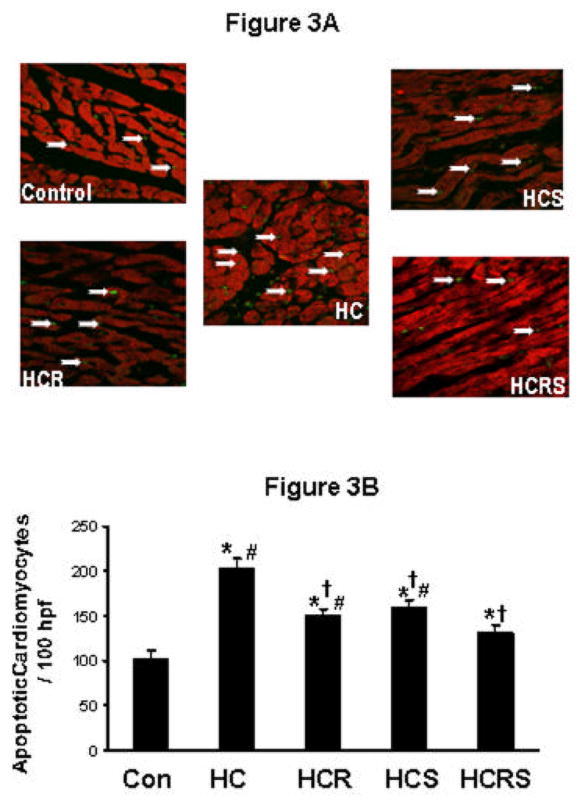
Cardiomyocyte apoptosis by TUNEL assay. Representative pictures show immunohistochemical staining of extended DNA. A: shows the extent of cardiomyocyte apoptosis between the comparative groups. B: shows the graphical representation of cardiomyocyte apoptosis. Data were expressed as a percentage of the total particular cell nuclei on counts per 100 high power fields. Values are mean ± Std. error (n=6). *P<0.05 compared to control, †P<0.05 compared to HC group, #P<0.05 compared to HCRS group. 100 hpf - represents 100X high power field.
Again, statin along with resveratrol achieved maximal therapeutic benefits as evidenced by significant protection of cardiomyocyte compared to monotherapy with statin or resveratrol.
Effect of resveratrol and statin on the phosphorylation of AKT and eNOS
Phosphorylated protein levels of Akt and eNOS were found to be decreased in HC group when compared to control. Resveratrol and statin treatment significantly enhanced the phosphorylation of Akt and e-NOS in all the treatment groups compared to the HC group (Figure 4A & 4B). The phosphorylation was more significant in combination group when compared to individual treatment groups.
Figure 4.
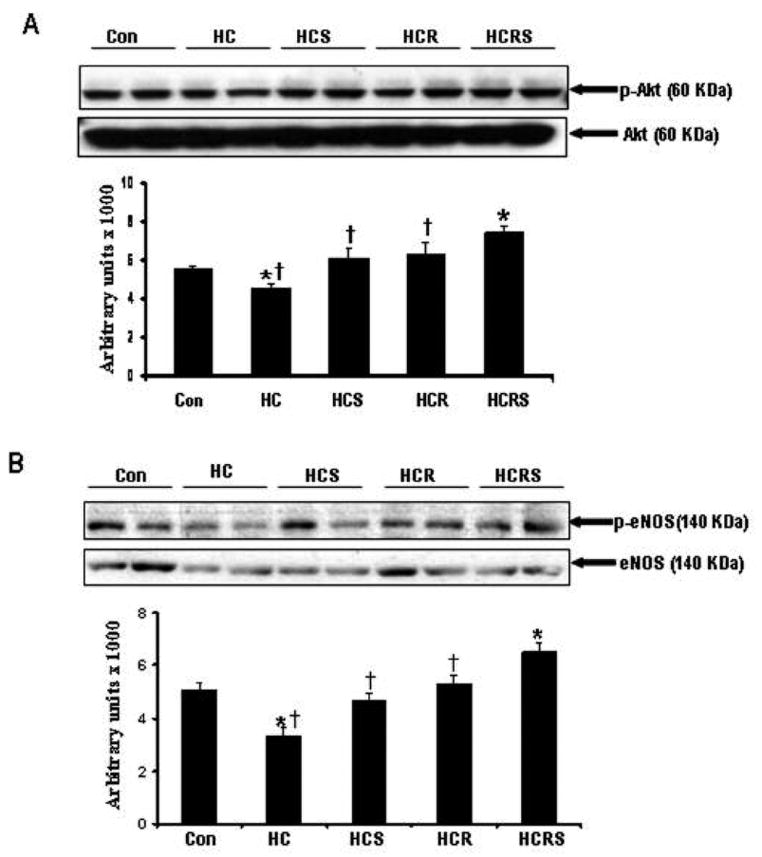
Representative Western blot showing the protein expression (A) Phosphorylated AKT and non-phosphorylated AKT. *P<0.05 compared to control, †P<0.05 compared to HCRS group (B) Phosphorylated eNOS and non-phosphorylated eNOS. *P<0.05 compared to control, †P<0.05 compared to HCRS group.
Effect of resveratrol and statin on β-catenin translocation
Significant nuclear translocation of β-catenin was observed in HCR, HCS and HCRS groups when compared to HC group after ischemia and reperfusion. The nuclear translocation of β-catenin was found to be more significant in HCR group when compared to HCS and HCRS groups (Figure 5). However, in combination group (HCRS) there is a moderate decrease in β-catenin translocation compared to HCR but it was significantly increased compared to the statin treated group.
Figure 5.

Representative immunohistochemical staining for β-catenin. Nuclei were counterstained with hematoxylin. Arrows indicate nuclear translocation of β-catenin between the comparative groups.
Effect of resveratrol and statin on mRNA levels of VEGF expression
Real time RT-PCR demonstrated increased expression of VEGF mRNA in HC (1.15fold), HCR (1.86fold), HCS (1.3fold) and HCRS (1.5fold) compared to control animals (Figure 6) indicating that mRNA level of VEGF expression is correlated with the extent of nuclear translocation of β-catenin. The resveratrol group has shown more expression of VEGF demonstrating the angiogenic property of resveratrol. Though statin and combination groups have shown increased expression of VEGF when compared to control and HC group, it was not significant like resveratrol. Again as other results, combination group documented more VEGF expression compared to statin therapy alone.
Figure 6.
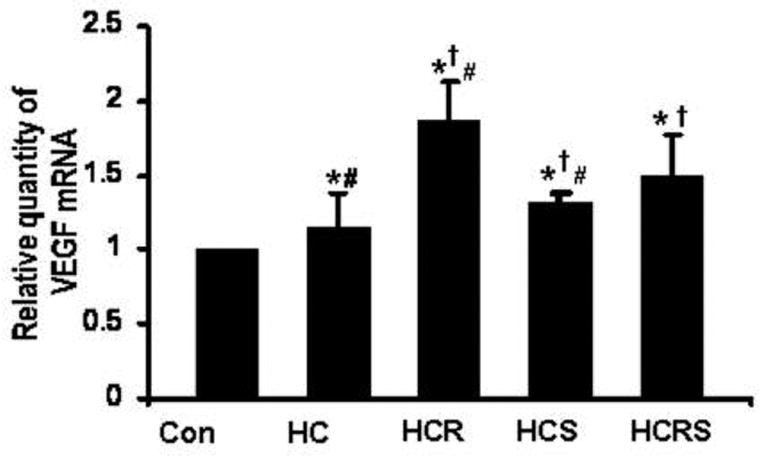
Graph represents Real Time RT-PCR analysis for VEGF mRNA between the comparative groups. *P<0.05 compared to control, †P<0.05 compared to hypercholesterol group, #P<0.05 compared to HCRS group.
Effect of resveratrol and statin on the extent of capillary density (CD-31)
At 400X magnification, 8 non-overlapping random fields, each selected from non-infarcted risk area were used for CD-31 counting. Four sections from each heart were examined. Increased capillary density was found in all the treatment groups when compared to control and HC. The increase was more prominent in HCR treated group when compared to HCS and HCRS group and correlated with the VEGF expression levels thus supporting the notion that the increased CD-31 in resveratrol treatment might be due to the angiogenic property of resveratrol (Figure 7). The combination therapy demonstrated significantly increased capillary density compared to statin but not compared to resveratrol.
Figure 7.
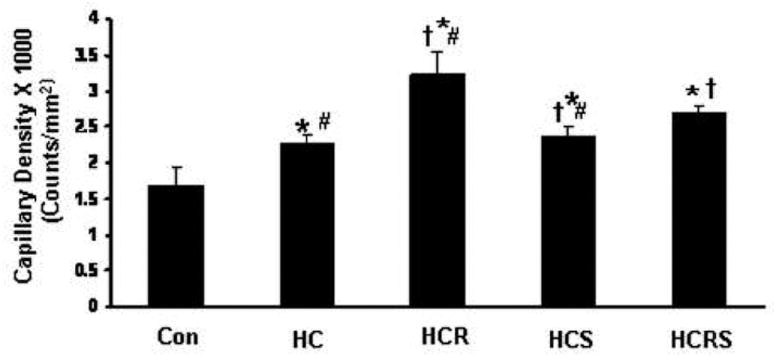
Effect of resveratrol and statin on capillary density. Quantitative analysis of capillary density in the peri-infarct area 7 days after Myocaridal Infarction. Results are expressed as mean ± Std. error of six hearts per group. *P<0.05 compared to control, †P<0.05 compared to hypercholesterol group, # P<0.05 compared to HCRS group.
Discussion
The present study demonstrates, for the first time, that high cholesterol diet-induced myocardial complications such as increased infarct size, extent of apoptosis, impaired angiogenesis could be controlled aggressively with dietary resveratrol supplementation. In addition we are able to demonstrate that statin along with resveratrol; a combination therapy is more beneficial to HC-myocardium compared to statin alone. One may speculate that rat may not be a proper model to test the effects of HC in–vivo. In our present study however, serum cholesterol level was almost double in HC rats compared to control. Moreover, several previous studies documented that rat models of HC represented and demonstrated alterations in vascular functions and microvascular reactivity to vasoactive substances [13, 14].
Hypercholesterolemia refers to levels of cholesterol in the blood that are normally higher than normal individual. Overwhelming evidence indicates that hypercholesterolemia and other lipid abnormalities provide an important modifiable risk factor for coronary heart disease (CHD). Risk of CHD increases progressively with higher levels of low-density lipoprotein (LDL) cholesterol while higher levels of high-density lipoprotein (HDL) cholesterol reduces the risk significantly. Furthermore, CHD is also found to be associated with coronary endothelial dysfunction and myocardial perfusion abnormalities [15]. Several clinical trials have demonstrated that statin therapy is effective for both the primary and secondary prevention of coronary artery diseases. It is well established that statins exert cardioprotective benefits by mechanisms independent of their serum lipid lowering effects that appear to be caused by improved endothelial function and NO synthesis. Moreover, it has been reported that statins can have both pro- and anti-angiogenic activities. It appears that low doses induce pro-angiogenic effects through Akt activation, which leads to eNOS phosphorylation and NO production [16]. Boodhwani et al has shown that atorvastatin at higher doses improves coronary endothelial dysfunction in hypercholesterolemic animals without improving angiogenic response [17]. Statins have also been shown to have dose dependent effects on VEGF expression [18]. The upregulation of eNOS-NO system by statin, has been proposed as a mechanism underlying how statins protect ischemic/reperfused myocardium [5, 19]. Despite several developments of novel approaches to cholesterol reduction, statin therapy remains the mainstay of cholesterol-lowering therapy. Therefore in literature both resveratrol and statin demonstrated cardioprotection through nitric oxide mechanism. However, recently we found that resveratrol, a polyphenolic compound present in red wine, along with lipid lowering property possesses several other additional properties. It significantly induces myocardial angiogenesis in a rat MI model through Trx-1, NO/HO-1 and VEGF mechanism [11]. Resveratrol is a fat-soluble compound that occurs in a trans and a cis configuration. Resveratrol has been found to inhibit the proliferation of vascular smooth muscle cells in culture [20]. The proliferation of vascular smooth muscle cells plays an important role in the progression of atherosclerosis [21]. In our previous study [12] resveratrol-mediated induction of Trx-1 shows sequential activation and expression of HO-1 as well as pro-angiogenic factor and cardioprotective molecule, VEGF in both in vitro and in vivo models. We have shown that adjunctive treatment with SnPP, inhibitor of HO-1 enzyme activity significantly reduces all the VEGF-induced angiogenic activities of resveratrol and Trx-1 in vitro and in vivo. In the present study however combination therapy was found to be very effective for HC myocardium compared to the statin therapy alone. The extent of Akt and eNOS phosphorylation was more drastic in the combination group than either statin or resveratrol alone. The fact that significant decrease in the extent of cardiomyocyte apoptosis was observed in the combination group compared to all other groups suggests that activation of Akt and eNOS plays a crucial role in acute cardioprotection against ischemia/reperfusion injury. It has been demonstrated that activation of Akt induces phosphorylation and inhibition of GSK-3β, which inhibits mitochondrial permeability transiton pore opening, a primary step for apoptotic and necrotic cell death [22]. On the other hand, activation of eNOS generates NO and activates mitochondrial KATP channels in a cGMP dependent manner, leading to acute cardioprotection [23]. Again, the following study suggests possible involvement of increased β-catenin translocation followed by VEGF expression in the resveratrol and combination groups after myocardial infarction which might be one of the positive effect of β-catenin and its angiogenic property that leads to chronic cardioprotection. From the literature β-catenin is found to be a critical mediator during development and angiogenesis [24], which is phosphorylated in a cytosolic multiprotein complex containing adenomatous polyposis coli protein (APC), Axin and GSK-3β [24–27]. When phosphorylation of β-catenin is blocked, this allows β-catenin to accumulate and translocate into nucleus, where it forms a complex with T-cell transcription factors/lymphoid-enhancer binding factor (TCF/LEF) family of transcription factors and is able to activate or repress several important target genes, such as c-Myc, cyclin D1, fibronectin, VEGF, Bcl-2 and survivin [28–31]. VEGF expression was found to be increased in HC (1.15 fold), HCR (1.86 fold), HCS (1.3 fold), HCRS (1.5 fold) when compared to control group. In this study again, the extent of angiogenesis measured by CD31 staining also validated strong angiogenic response of resveratrol compared to any other treatment groups. However, increase in capillary density in HC might be a compensatory response aimed at sustaining myocardial perfusion. The ability of resveratrol and statin treatments alone or in combination improve both myocardial microvascular architecture and function. Therefore, enhanced neo-vascularization in the infarcted myocardium induced by statin and resveratrol seems to mediate chronic cardioprotection against ventricular remodeling. Furthermore, the effect of resveratrol as well as statin may be dose-dependent, because both have biphasic effects on angiogenesis. The dose used for statin (1mg/kg bw) in our experiments more or less is found to be non-angiogenic where as the dose used for resveratrol is angiogenic. The above mentioned molecular adaptations may have influence also the growth of resident cardiac progenitor cells and amplify myocytes most importantly its proliferation which might have contributed in the reduction of infarct size in the treatment groups. However, this area of work is under investigation and most probably more new mechanisms will help us to explain the phenomena with which we are actively involved at present.
Therefore, our results clearly demonstrated for the first time that statin in combination with resveratrol may be more effective or beneficial during the ischemic scenario than statin alone. Further biochemical and pharmacological investigations are in progress in our laboratory to elucidate in detail the mechanism of action of single and or combination therapy in ischemic myocardium.
Table 1.
EFFECT OF RESVERATROL AND STATIN ON LIPID LEVELS
| S.No | Groups | Cholesterol (mg/dl) | Triglycerides (mg/dl) | HDL-C (mg/dl) | LDL-C (mg/dl) |
|---|---|---|---|---|---|
| 1 | Control | 86.34±3.38 | 78.83±2.34 | 41.67±3.62 | 29.00±3.21 |
| 2 | HC | 142.83±4.16* | 150.67±3.14* | 26.65±2.80* | 86.03±4.70* |
| 3 | HCR | 101.16±3.18*† | 96.67±2.74*† | 34.00±1.68*† | 47.83±2.93*† |
| 4 | HCS | 94.16±7.14† | 92.83±2.48*† | 41.83±2.31†# | 33.76±4.21†# |
| 5 | HCRS | 87.66±4.22†# | 85.00+2.82*†#Ψ | 42.00±3.22†# | 28.67±2.34†# |
Results are expressed as Means ± SD.
p<0.05, compared with group 1;
p<0.05, compared with group 2;
p<0.05, compared with group 3;
<0.05, compared with group 4
Acknowledgments
This study was supported by National Institutes of Health Grants HL 56803, HL 69910 and HL 85804.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stamler J, Daviglus ML, Garside DB, Dyer AR, Greenland P, Neaton JD. Relationship of baseline serum cholesterol levels in 3 large cohorts of younger men to long-term coronary, cardiovascular, and all-cause mortality and to longevity. JAMA. 2000;284:311–318. doi: 10.1001/jama.284.3.311. [DOI] [PubMed] [Google Scholar]
- 2.Vogel RA, Corretti MC, Gellman J. Cholesterol, Cholesterol lowering, and endothelial function. Prog in Cardiovasc diseases. 1998;41(2):117–136. doi: 10.1016/s0033-0620(98)80008-x. [DOI] [PubMed] [Google Scholar]
- 3.Gylling H. Cholesterol metabolism and its implications for therapeutic interventions in patients with hypercholesterolaemia. In J Clin Pract. 2004;58(9):859–866. doi: 10.1111/j.1742-1241.2004.00351.x. [DOI] [PubMed] [Google Scholar]
- 4.Futterman LG, Lemberg L. Statin pleiotropy: fact or fiction? Am J Crit Care. 2004;13:244–249. [PubMed] [Google Scholar]
- 5.Bell RM, Yellon DM. Atorvastatin, administered at the onset of reperfusion, and independent of lipid lowering, protects the myocardium by up-regulating a pro-survival pathway. J Am Col Cardiol. 2003;41:508–515. doi: 10.1016/s0735-1097(02)02816-4. [DOI] [PubMed] [Google Scholar]
- 6.Ferrero ME, Bertelli AE, Fulgenzi A, Pellegatta F, Corsi MM, Bonfrate M, Ferrara F, De Caterina R, Giovannini L, Bertelli A. Activity in vitro of resveratrol on granulocyte and monocyte adhesion to endothelium. Am J Clin Nutr. 1998;68:1208–1214. doi: 10.1093/ajcn/68.6.1208. [DOI] [PubMed] [Google Scholar]
- 7.Imamura G, Bertelli AA, Bertelli A, Otani H, Maulik N, Das DK. Pharmacological preconditioning with resveratrol : an insight with iNOS knockout mice. Am J Physiol Heart Circ Physiol. 2002;282:H1996–H2003. doi: 10.1152/ajpheart.01013.2001. [DOI] [PubMed] [Google Scholar]
- 8.Kaga S, Zhan Lijun, Altaf Elham, Maulik Nilanjana. Glycogen synthase kinase-3β/β-catenin promotes angiogenic and anti-apoptotic signaling through the induction of VEGF, Bcl-2 and survivin expression in rat ischemic preconditioned myocardium. J Mol Cell Cardiol. 2006;40(1):138–147. doi: 10.1016/j.yjmcc.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Thirunavukkarasu M, Penumathsa SV, Juhasz B, Zhan L, Cordis G, Altaf E, et al. Niacin bound chromium enhances myocardial protection from ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2006;291:H820–826. doi: 10.1152/ajpheart.00134.2006. [DOI] [PubMed] [Google Scholar]
- 10.Hochhauser E, Kivity S, Offen D, Maulik N, Otani H, Barhum Y, Pannet H, Shneyvays V, Shainberg Goldshtaub V, Tobar A, Vidne BA. Bax ablation protects against myocardial ischemia-reperfusion injury in transgenic mice. Am J Physiol Heart Circ Physiol. 2003;284 (6):H2351–2359. doi: 10.1152/ajpheart.00783.2002. [DOI] [PubMed] [Google Scholar]
- 11.Fukuda S, Kaga S, Zhan L, Bagchi D, Das DK, Bertelli A, Maulik N. Resveratrol ameliorates myocardial damage by inducing vascular endothelial growth factor-angiogenesis and tyrosine kinase receptor Flk-1. Cell Biochem Biophys. 2006;44(1):43–49. doi: 10.1385/CBB:44:1:043. [DOI] [PubMed] [Google Scholar]
- 12.Kaga S, Zhan lijun, Matsumoto Masahiko, Maulik Nilanjana. Resveratrol enhances neovascularization in the infarcted rat myocardium through the induction of thioredoxin-1, heme-oxygenase-1 and vascular endothelial growth factor. J Mol and Cell Cardiol. 2005;39:813–822. doi: 10.1016/j.yjmcc.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Nunnari JJ, Zand T, Joris I, Majno G. Quantitation of oil red O staining of the aorta in hypercholesterolemic rats. Exper Mol Pathol. 1989;51(1):1–8. doi: 10.1016/0014-4800(89)90002-6. [DOI] [PubMed] [Google Scholar]
- 14.Schuschke DA, Joshua IG, Miller FN. Comparison of early microcirculatory and aortic changes in hypercholesterolemic rats. Arter Throm. 1991;11(1):154–160. doi: 10.1161/01.atv.11.1.154. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez-Porcel M, Lerman A, Best PJ, Krier JD, Napoli C, Lerman LO. Hypercholesterolemia impairs myocardial perfusion and permeability:role of oxidative stress and endogenous scavenging activity. J Am Col Cardiol. 2001;37(2):608–615. doi: 10.1016/s0735-1097(00)01139-6. [DOI] [PubMed] [Google Scholar]
- 16.Skaletz-Rorowski A, Walsh K. Statin therapy and angiogenesis. Current Opinion Lipidol. 2003;14(6):599–603. doi: 10.1097/00041433-200312000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Boodhwani M, Nakai Y, Voisine P, Feng J, Li J, Mieno S, et al. High-Dose atorvastatin improves hypercholesterolemic coronary endothelial dysfunction without improving the angiogenic response. Circ. 2006;114:I402–I408. doi: 10.1161/CIRCULATIONAHA.105.000356. [DOI] [PubMed] [Google Scholar]
- 18.Urbich C, Dernbach E, Zeiher AM, Dimeller S. Double-edged role of statins in angiogenesis signaling. Circ Res. 2002;90:737–744. doi: 10.1161/01.res.0000014081.30867.f8. [DOI] [PubMed] [Google Scholar]
- 19.Di Napoli P, Antonio Tacardi A, Grilli A, Spina R, Fecalo M, Barsotti A, De Caterina R. Simvastatin reduces reperfusion injury by modulating nitric oxide synthase expression: an ex vivo study in isolated working rat hearts. Cardiovasc Res. 2001;51(2):283–293. doi: 10.1016/s0008-6363(01)00306-6. [DOI] [PubMed] [Google Scholar]
- 20.Poussier B, Cordova AC, Becquemin JP, Sumpio BE. Resveratrol inhibits vascular smooth muscle cell proliferation and induces apoptosis. J Vasc Surg. 2005;42:1190–1197. doi: 10.1016/j.jvs.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 21.Ross R. Atherosclerosis – an inflammatory disease. New Eng J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 22.Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, Ziman BD, Wang S, Ytrehus K, Antos CL, Olson EN, Sollott SJ. Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest. 2004;113(11):1535–1549. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Costa AD, Garlid KD, West IC, Lincoln TM, Downey JM, Cohen MV, Critz SD. Protein kinase G transmits the cardioprotective signal from cytosol to mitochondria. Circ Res. 2005;97(4):329–336. doi: 10.1161/01.RES.0000178451.08719.5b. [DOI] [PubMed] [Google Scholar]
- 24.Klinge CM, Blankenship KA, Risinger KE, Bhatnager S, Noisin EL, Sumanasekara WK, Zhao L, Brey DM, Keynton RS. Resveratrol and estradiol rapidly activate MAPK signaling through estrogen receptors alpha and beta in endothelial cells. J Biol Chem. 2005;280(9):7460–7468. doi: 10.1074/jbc.M411565200. [DOI] [PubMed] [Google Scholar]
- 25.Pozo-Guisado E, Lorenzo-Benayas MJ, Fernandez-Salguero PM. Resveratrol modulates the phosphoinositide 3-Kinase pathway through an estrogen receptor alpha-dependent mechanism: relevance in cell proliferation. Int J Cancer. 2004;109:167–173. doi: 10.1002/ijc.11720. [DOI] [PubMed] [Google Scholar]
- 26.Bertelli AA, Giovannini L, Bernini W, Migliori M, Fregoni M, Bavaresco L, Bertelli A. Antiplatelet activity of cis-resveratrol. Drug under Exper Clin Res. 1996;22:61–66. [PubMed] [Google Scholar]
- 27.Bianchini F, Vainio H. Wine and resveratrol: mechanisms of cancer prevention? E J Can Prev. 2003;12:417–425. doi: 10.1097/00008469-200310000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Lin MT, Yen ML, Lin CY, Kuo ML. Inhibition of vascular endothelial growth factor-induced angiogenesis by resveratrol through interruption of Src-dependent vascular endothelial cadherin tyrosine phosphorylation. Mol Pharmacol. 2003;64:1029–1036. doi: 10.1124/mol.64.5.1029. [DOI] [PubMed] [Google Scholar]
- 29.Cao Z, Fang J, Xia C, Shi X, Jiang BH. trans-3,4,5′-Trihydroxystibene inhibits hypoxia –inducible factor 1 alpha and vascular endothelial growth factor expression in human ovarian cancer cells. Clin Can Res. 2004;10:5253–5263. doi: 10.1158/1078-0432.CCR-03-0588. [DOI] [PubMed] [Google Scholar]
- 30.Kaneuchi M, Sasaki M, Tanaka Y, Yamamoto R, Sakuragi N, Dahiya R. Resveratrol suppresses growth of Ishikawa cells through down-regulation of EGF. Int J Oncol. 2003;23:1167–1172. [PubMed] [Google Scholar]
- 31.Khanna S, Roy S, Bagchi D, Bagchi M, Sen CK. Upregulation of oxidant induced VEGF expression in cultured keratinocytes by a grape seed proanthocyanidin extract. Free Rad Biol Med. 2001;31:38–42. doi: 10.1016/s0891-5849(01)00544-5. [DOI] [PubMed] [Google Scholar]


