Abstract
A commercially available Argonaut VacMaster-96™ plate-to-plate solid-phase extraction (SPE) station equipped with twenty four FluoroFlash® cartridges is employed for parallel purification of fluorous reaction mixtures. Each cartridge charged with 3 g of fluorous silica gel has the capability to produce up to 100 mg of purified small molecules. The 24-well receiving plate has a standard footprint that can be directly concentrated in a Genevac vacuum centrifuge. Important issues such as sample loading, product cross-contamination, cartridge reuse, and reproducibility are investigated. The SPE system has been demonstrated in the purification of three small libraries that were produced involving amine scavenging reactions with fluorous isatoic anhydride, amide coupling reactions with 2-chloro-4,6-bis[(perfluorohexyl)propyloxy]-1,3,5-triazine (fluorous CDMT), and amide coupling reactions with a newly developed fluorous Mukaiyama condensation reagent.
Introduction
FluoroFlash® silica gel has a perfluorooctylethylsilyl (-Si(CH3)2CH2CH2C8F17) stationary phase. Solid-phase extraction (SPE) cartridges and HPLC columns packed with fluorous silica gel can be used for separations of highly fluorinated (fluorous) molecules from non-fluorous molecules.1–3 Compared to liquid-liquid extraction with fluorous solvents such as FC-72 (perfluorohexanes),4 fluorous silica gel has the capability to separate compounds with significantly lower fluorine content. A small molecule attached to a C6F17 or C8F17 tag usually has sufficient retention on a FluoroFlash® SPE cartridge for convenient separation from nontagged molecules. Since the Curran group introduced fluorous SPE (F-SPE) for “light fluorous” synthesis in 1997,5 this technique has been widely used in the purification of reaction mixtures containing fluorous catalysts,6 scavengers,7 reagents,8 protecting groups,9,10 and even biopolymers such as proteins and DNA fragments.11 However, F-SPE separations described in literature were conducted with a single cartridge or on a 2x12 SPE vacuum manifold;12 none of them was in a plate format. Since plate-to-plate SPE for sample preparation is a well established technology,13 we decided to incorporate it into fluorous synthesis to improve the efficiency of F-SPE.
Results and Discussion
1. Evaluation of Plate-To-Plate SPE Separation System
Commercially available plate-to-plate SPE vacuum manifolds from United Chemical Technologies, Inc. (UTC), Supelco, Waters, and Argonaut have similar designs. We selected the Argonaut VacMaster-96™ system because it has a modest price and good vacuum seal (Figure 1).14 The vacuum is applied by connecting the outlet from the receiving plate to a vacuum pump.15 Whatman® receiving plates were used in this project.16 These plates have a standard footprint and are configured in 24-, 48-, and 96-well formats. We selected the 24-well plate because it has the capability to purify a set of twenty-four compounds in 10–100 mg quantity, which is suitable for analog synthesis in common medicinal chemistry programs. This plate system has following technical features: 1) each 6 mL cartridge can be charged with 3 g of fluorous silica gel17 leaving enough space to fill with elution solvents; 2) each well in the receiving plate has a 10 mL volume to collect fractions; and 3) the receiving plate can be directly concentrated in a Genevac vacuum centrifuge.
Figure 1.
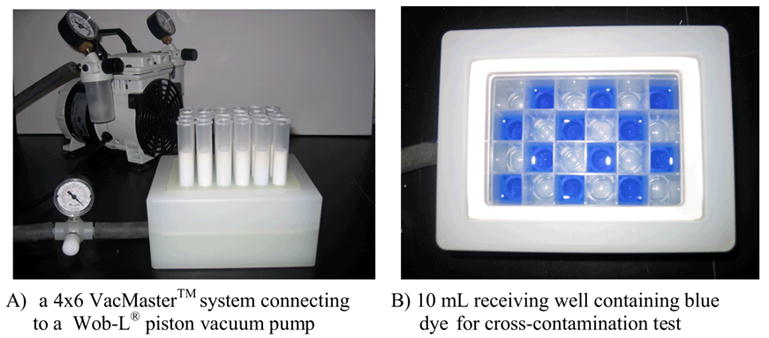
Plate-To-Plate SPE System
To evaluate the VacMaster-96™ system for F-SPE, we first tested the possibility of cross-contamination that could be caused by a sample splashing from the cartridge tip to the adjacent wells in the receiving plate. Every other cartridge on the plate was charged with a nonfluorous blue dye18 solution and eluted with 2x3 mL of 80:20 MeOH-H2O. Visual inspection of the receiving plate indicated no cross-contamination (Figure 1, B).
Scheme 1.
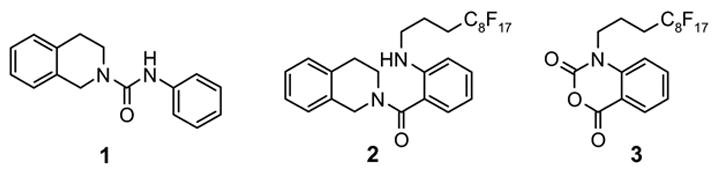
A mixture sample for SPE test
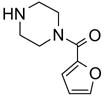
We next evaluated the sample loading level by using a mixture generated from an amine scavenging reaction with fluorous isatoic anhydride 3 (Scheme 1).7d The mixture contained urea 1, scavenged amine 2, and excess scavenger 3 in a molar ratio of 5:1:1. The cartridges were preconditioned with 80:20 MeOH/H2O (2x3 mL). After the sample was loaded, each cartridge was eluted with 80:20 MeOH/H2O (2x3 mL). In the mass loading test, the loading solvent (THF) and volume (0.3 mL) were fixed, and different amounts of the sample were tested at 12, 24, 36, 48, 90, and 150 mg. In all cases, no fluorous sample breakthrough was observed in the 80:20 MeOH-H2O fractions. This result indicated a 3 g cartridge can be loaded with up to 150 mg (5% mass loading) of a mixture sample.
At 1% mass loading, different loading solvents including dichloromethane (DCM), tetrahydrofuran (THF), and N,N-dimethylformamide (DMF) were also tested at four different volumes: 0.2, 0.4, 0.6 and 0.8 mL. A small amount (<5%) of fluorous samples 2 and 3 were leached to the MeOH/H2O fraction when 0.4 mL DCM was used as the loading solvent. More fluorous sample (~10%) was leached when 0.4 mL of THF was used as the loading solvent. In contrary, no fluorous sample leaching was observed even upto 0.8 mL of DMF. These results are consistent with the solvent fluorophilicity order: THF > DCM ≫ DMF. F-SPE is a fluorine-fluorine affinity, rather than a mass-controlled separation process. It is sensitive to the volume of the loading solvent, especially with fluorophillic solvents such as THF and DCM. The selection of a right loading solvent and use of an appropriate volume is critical to the success of F-SPE. For a 3 g FluoroFlash® cartridge and mass loading less than 5%, we suggest following loading solvents and volumes: THF < 0.30 mL, DCM < 0.35 mL, and DMF < 0.8 mL.
Reuse of fluorous silica gel cartridges is important to reduce cost of consumables and to minimize waste disposal. We prepared six reaction mixtures 5a–f from fluorous isatoic anhydride scavenging reactions (Table 1).7d Six samples were each divided to ten portions and subjected to ten rounds of SPE on different cartridges. In each round, six samples were loaded onto six cartridges and eluted with 80:20 MeOH/H2O (2x3 mL) to collect the product fraction. The plate was concentrated and each product was analyzed by LCMS. A new receiving plate was used to collect MeOH (2x3 mL) fractions containing fluorous components. The cartridge plate was then washed with acetone (2x3 mL) and pre-conditioned with 80:20 MeOH/H2O (2x3 mL) for next round of SPE. The results of ten rounds of SPE using reconditioned cartridges are listed in Table 1. Product purities determined by LCMS (UV254) were greater than 90%. We noticed compounds 5d, 5e and 5f had relatively large deviations (~7 mg, 30–35% of the expected yield). That was partially due to the solubility of these mixtures in concentrated loading solvent (THF), which generated a small amount of precipitation in the sample stock solution and thus affected the reproducibility of sample loading.
Table 1. SPE with reconditioned cartridgesa.
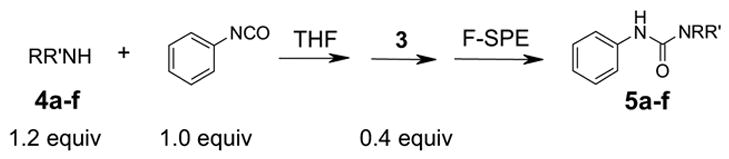
| NRR' =-NHBu |

|
|
|
|
|
|
| run | 5a | 5b | 5c | 5d | 5e | 5f |
| 1 | 11mg | 11mg | 15mg | 15mg | 14mg | 13mg |
| 2 | 12 | 11 | 16 | 15 | 16 | 19 |
| 3 | 11 | 11 | 15 | 14 | 16 | 18 |
| 4 | 12 | 9 | 16 | 14 | 16 | 19 |
| 5 | 13 | 13 | 15 | 16 | 17 | 19 |
| 6 | 12 | 11 | 15 | 15 | 15 | 17 |
| 7 | 15 | 11 | 15 | 15 | 16 | 18 |
| 8 | 11 | 10 | 16 | 10 | 16 | 13 |
| 9 | 12 | 9 | 16 | 16 | 16 | 19 |
| 10 | 15 | 12 | 18 | 17 | 11 | 20 |
1% sample loading in THF (0.25 mL), samples were collected in 80:20 MeOH/H2O (2x3 mL) fractions, cartridges were washed with MeOH (2x3 mL), acetone (2x3 mL), and then reconditioned with 80:20 MeOH/H2O (2x3 mL) for next runs.
Plate-To-Plate SPE Separation for Parallel Synthesis
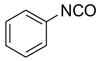
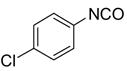
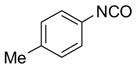
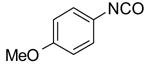
After conducting sample loading, cross-contamination, and cartridge reuse studies, we next evaluated the separation of three demonstration libraries. The first library was the preparation of ureas 6 by reaction of an array of four aryl isocyanates with six amines.7d Amines were used in slight excess (1.2 equiv). Unreacted amines were scavenged with fluorous isatoic anhydride 3 (0.4 equiv). The twenty-four reaction mixtures were directly loaded onto a pre-conditioned SPE cartridge plate. The cartridges were first eluted with 80:20 MeOH/H2O (2x5 mL) to provide the target ureas followed by MeOH or acetone (2x5 mL) to clean the cartridges. The results listed in Table 2 show that product yields are greater than 79% with purities greater than 90%, with the exception of only one product.
Table 2.
Ureas generated from amine scavenging reactions with fluorous isatoic anhydride 3
| H2NBu |
|
|
|
|
|
|
|
95(92)* | 83(96) | 95(93) | 86(96) | 92(91) | 100(96) |
|
83(100) | 81(99) | 79(100) | 81(100) | 83(100) | 90(100) |
|
100(80) | 100(96) | 100(97) | 100(94) | 100(96) | 100(99) |
|
100(94) | 100(99) | 100(100) | 100(100) | 100(100) | 100(100) |
yield% (purity%), purities were determined by LCMS with UV254 detection
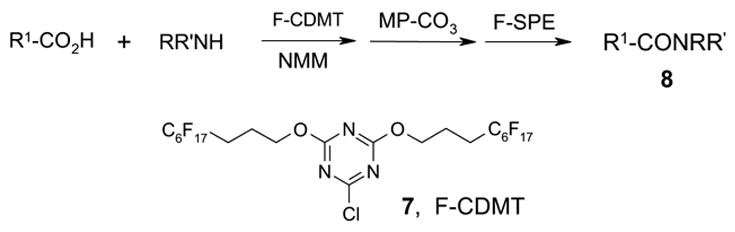
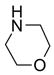
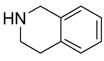
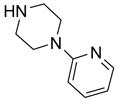
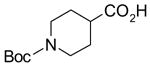
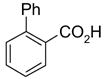
In a recent publication, Dembinski and coworkers reported the preparation of 2-chloro-4,6-bis[(perfluorohexyl)propyloxy]-1,3,5-triazine 7 (fluorous CDMT) and its use as a coupling reagent in the peptide synthesis.19 We employed the same reagent for amide coupling reactions. Four representative carboxylic acids and six amines were selected for 24 parallel reactions. The coupling reactions were conducted in the presence of N-methylmorpholine (NMM) using DCM as the reaction solvent. After the reactions were over, a macroporous polystyrene anion-exchange resin (MP-CO3)20 was added to free the NMM salt. Reaction mixtures were filtered and filtrates were concentrated to dryness and then loaded to SPE cartridge plate with 0.3 mL of DMF. Following general procedures for plate SPE purification, 24 amides were collected in a single receiving plate for concentration and analysis. Results listed in Table 3 show product yields are in the range of 34–100% and purities are 60–100%.
Table 3.
Amide coupling products generated with F-CDMT 7 as a coupling agent
|
|
|
|
|
|
|
|
|
81(100)* | 50(83) | 57(91) | 80(90) | 100(60) | 67(100) |
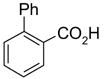
|
68(99) | 45(88) | 53(95) | 70(100) | 84(95) | 62(93) |
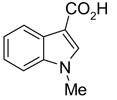
|
36(99) | 37(92) | 34(100) | 67(97) | 76(92) | 47(95) |
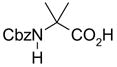
|
58(81) | 58(81) | 58(92) | 45(90) | 63(77) | 37(99) |
yield% (purity%), purities were determined by LCMS with UV254 detection
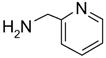
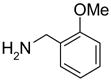
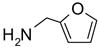
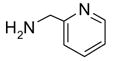
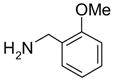
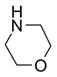
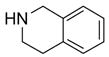
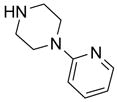
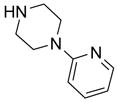
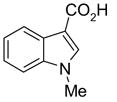
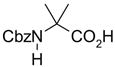
In another amide coupling reaction, we employed the newly developed pyridinium salt 9 as a fluorous version of the Mukaiyama reagent.21 The coupling reactions were conducted using the same set of substrates described in Table 3. Mixtures of fluorous pyridinium salt (1.3 equiv), carboxylic acid (1.2 equiv), 1-hydroxybenzotriazole (HOBt, 1.3 equiv), and DIEA (4.0 equiv) were reacted in THF for 10 min to form OBt esters. Amines (1.0 equiv) were added and the mixtures were stirred for 1 h. N-Methyl-[3-(perfluorononyl)propyl]amine (0.4 equiv) in THF was then added to scavenge excess OBt esters. After 12 h, MP-CO3 (10 equiv) were added and the mixture was stirred for 3 h. After filtration, the filtrates were concentrated and loaded onto the SPE cartridge plate with 0.3 mL of DCM. Following general procedures for plate SPE purification, 24 amides were collected in a single receiving plate for concentration and analysis. The results listed in Table 4 shows most products have good to excellent yields and greater than 95% purities. Even though the procedures for reactions with F-CDMT 7 are simple because no scavenging step is needed, a direct comparison of the results reported in Tables 3 and 4 clearly demonstrates that coupling reactions with fluorous pyridinium salt 9 are obtained with both higher yields and purities.22
Table 4.
Amide coupling products generated with 9 as a coupling agent
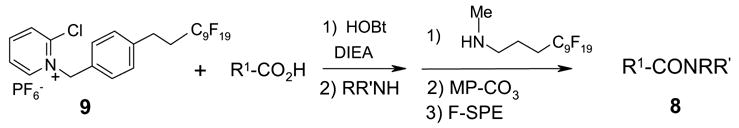
|
|
|
|
|
|
|
|
98(99)* | 82(97) | 89(99) | 98(99) | 91(97) | 93(99) |
|
95(99) | 84(98) | 96(99) | 93(99) | 96(98) | 99(99) |
|
40(99) | 66(99) | 20(98) | 95(99) | 95(99) | 93(99) |
|
88(86) | 87(75) | 87(75) | 80(99) | 80(99) | 46(99) |
yield% (purity%), purities were determined by LCMS with UV210 detection
In summary, a plate-to-plate SPE system consisting of a VacMaster-96™ SPE station, 24-channel plates, and FluoroFlash® cartridges has been developed for parallel separation of fluorous reaction mixtures. This system is suitable for purification of twenty-four reactions producing up to 100 mg of product. Important issues such as sample loading capability and cartridge reuse have been investigated. Loading samples to fluorous silica gel packed in deep-well SPE plates instead of parallel-plugged cartridges and conducting SPE with 48- and even 96-well plates are currently under active investigation and will be reported in due course.
Experimental Section
General Methods
All fluorous reagents and cartridges are available from Fluorous Technologies, Inc.17 Other reagents and solvents were obtained from commercial sources. SPE purifications were conducted on an Argonaut VacMaster SPE manifold with Isolute Array-24 cartridge plate.15 Whatman® 24-well plates were used as receiving plate.17 LCMS spectra were obtained on an Agilent 1100 system. Genevac EZ-2 vacuum centrifuge was used for solvent evaporation. The purities of products were determined by LCMS with a C18 column.
General Procedures for Plate-to-Plate SPE
Fluorous SPE cartridges were washed with acetone or MeOH (3 mL) followed by 80:20 MeOH/H2O (2x3 mL). Sample mixtures in minimum amount of solvent were loaded onto the cartridges with a 6 channel pipette. The cartridges were first eluted with 80:20 MeOH/H2O (2x5 mL) for desired product followed by MeOH or acetone (2x5 mL) wash to clean the cartridges. The elution speed was controlled by adjusting the vacuum (5–10 mmHg) to allow ~1 inch/min movement of the solvent. The MeOH/H2O fraction plates were concentrated in Genevac EZ-2 vacuum centrifuge. The products were transferred to pre-weighed 1 dram vials with DCM and concentrated on Genevac EZ-2 vacuum centrifuge.
General Procedures for Parallel Urea Formation Reactions (Table 2)
Isocyanates each in THF (0.6 mL) were distributed into a row of 6 vials (0.1 mL for each vial) sitting in a 24-well plate. Six amines in THF (0.4 mL) were distributed into a column of 4 vials (0.1 mL for each vial). The reaction mixtures were shaken at 600 rpm for 1 h, and then fluorous isatoic anhydride 3 in THF (0.1 mL) were added to each vial. The reaction mixtures were shaken at 600 rpm for 2 h at room temperature. Each mixture in 0.3 mL of THF was warmed to 40 °C and loaded onto SPE cartridges pre-conditioned with 80:20 MeOH/H2O (2x3 mL). Standard SPE procedures were performed and the MeOH/H2O fraction plates were concentrated in a Genevac EZ-2 vacuum centrifuge. The products were transferred to pre-weighed 1 dram vials with DCM, concentrated on Genevac EZ-2 vacuum centrifuge, and weighed. All the products were analyzed by LCMS.
General Procedures for Parallel Amide Coupling Reactions using F-CDMT 7 (Table 3)
Carboxylic acids and NMM in DCM (1.2 mL) were distributed into 4 rows of 1 dram vials sitting in a 24 well plate. Fluorous CDMT 7 in DCM (4.8 mL) was added to each vial and the reaction mixtures were shaken at 600 rpm for 20 min. Six amines in THF (0.8 mL) were distributed into the columns of the vials. The reaction mixtures were shaken at 600 rpm over night before MP-CO3 was added to each vial. The reaction mixtures were shaken at 600 rpm for 2 h at room temperature and transferred into a 24 well filtration plate. The beads were washed with DCM (3x1 mL) and the filtrates in the receiving plate were concentrated on Genevac EZ-2 vacuum centrifuge. The residues were dissolved in DMF (0.4 mL) and loaded onto fluorous SPE cartridges which were preconditioned with 80:20 MeOH/H2O (2x3 mL). Standard SPE procedures were performed, and the plate had the MeOH/H2O fraction was concentrated on Genevac EZ-2 vacuum centrifuge. The products were transferred to pre-weighed 1 dram vials with DCM, concentrated on Genevac EZ-2 vacuum centrifuge and weighed. All products were analyzed by LCMS.
General Procedures for Amide Coupling Reactions (0.1 mmol Scale) using Fluorous Pyridinium Salt (Table 4)
To each vial containing a mixture of carboxylic acid (0.72 mmol), HOBt (0.78 mmol), and pyridinium salt 9 (0.78 mmol) was added THF (5 mL) and N,N-diisopropylethylamine (0.41 mL, 2.3 mmol). The vials were shaken at 600 rpm for 10 min. The total volume of the solution was adjusted to 6.0 mL by adding THF. The solution was split into 6 (1 mL each), and each solution was mixed with a solution of an amine (0.1 mmol) in THF (1 mL). The reactions were monitored by LCMS. Upon disappearance of the amine, a solution of N-methyl-[3-(perfluorononyl)propyl]amine in THF (0.08 M, 0.5 mL, 0.04 mmol) was added. Upon disappearance of the active ester (HOBt ester) intermediate (LC-MS analysis), MP-carbonate (loading = 3.4 mmol/g, 0.45 g, 1.5 mmol) was added. The mixture was shaken at 600 rpm for 3 h, the resin was then filtered off, and rinsed with THF (3x2 mL). The filtrates were combined and concentrated on a Genevac EZ-2 vacuum centrifuge. Each sample was dissolved in DCM (0.3 mL) (in the case where product did not fully dissolve in DCM, a small amount of DMF was added to improve solubility). Each solution was loaded onto a SPE cartridge preconditioned with 80:20 MeOH/H2O (2x3 mL). Standard SPE procedure was performed as described above, and the MeOH/H2O fraction plates were concentrated on Genevac EZ-2 centrifuge. Each product was then dissolved, transferred to a pre-weighed vial, concentrated, and weighed. All the products were analyzed by LCMS for purity assessment.
Acknowledgments
This work was financially supported by National Institutes of General Medical Sciences SBIR grant (2R44GM067326-02A1).
References and notes
- 1.General reviews on fluorous silica gel-based separations: Curran DP. Synlett. 2001:1488.Curran DP. In: Handbook of Fluorous Chemistry. Gladysz JA, Curran DP, Horvath IT, editors. Wiley-VCH; Weinheim: 2004. pp. 101–127.
- 2.Recent reviews on fluorous synthesis: Curran DP. ref. 1b. :128–155.Zhang W. Chem Rev. 2004;104:2531. doi: 10.1021/cr030600r.Fache F. New J Chem. 2004;28:1277.Zhang W. Tetrahedron. 2003;59:4475.Pozzi G, Shepperson I. Coord Chem Rev. 2003;242:115.Dobbs AP, Kimberley MR. J Fluorine Chem. 2002;118:3.Tzschucke CC, Markert C, Bannwarth W, Roller S, Hebel A. Angew Chem Int Ed. 2002;41:3964. doi: 10.1002/1521-3773(20021104)41:21<3964::AID-ANIE3964>3.0.CO;2-3.
- 3.Fluorous HPLC separations: Curran DP, Luo Z. J Am Chem Soc. 1999;121:9069.Curran DP, Oderaotoshi Y. Tetrahedron. 2001;57:5243.Luo Z, Zhang Q, Oderaotoshi Y, Curran DP. Science. 2001;291:1766. doi: 10.1126/science.1057567.Zhang W, Luo Z, Chen CHT, Curran DP. J Am Chem Soc. 2002;124:10443. doi: 10.1021/ja026947d.
- 4.(a) Gladysz JA, Emnet C. ref. 1b. :11–23. [Google Scholar]; (b) Gladysz JA. ref. 1b. :41–55. [Google Scholar]
- 5.Curran DP, Hadida S, He M. J Org Chem. 1997;62:6714. [Google Scholar]
- 6.(a) Matsugi M, Curran DP. J Org Chem. 2005;70:1636. doi: 10.1021/jo048001n. [DOI] [PubMed] [Google Scholar]; (b) Curran DP, Fischer K, Moura-Letts G. Synlett. 2004:1379. [Google Scholar]
- 7.(a) Zhang W, Curran DP, Chen CHT. Tetrahedron. 2002;58:3871. [Google Scholar]; (b) Lindsley CW, Zhao Z, Leister WH. Tetrahedron Lett. 2002;43:4225. [Google Scholar]; (c) Lindsley CW, Zhao Z, Leister WH, Strauss KA. Tetrahedron Lett. 2002;43:6319. [Google Scholar]; (d) Zhang W, Chen CHT, Nagashima T. Tetrahedron Lett. 2003;44:2065. [Google Scholar]; (e) Werner S, Curran DP. Org Lett. 2003;5:3293. doi: 10.1021/ol035214a. [DOI] [PubMed] [Google Scholar]
- 8.(a) Dobbs AP, McGregor-Johnson C. Tetrahedron Lett. 2002;43:2807. [Google Scholar]; (b) Dandapani S, Curran DP. Tetrahedron. 2002;58:3855. [Google Scholar]; (c) Lindsley CW, Zhao Z, Newton RC, Leister WH, Strauss KA. Tetrahedron Lett. 2002;43:4467–4470. [Google Scholar]; (d) Zhang W, Chen CHT, Lu Y, Nagashima T. Org Lett. 2004;6:1473–1476. doi: 10.1021/ol0496428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Review on fluorous protecting groups and tags: Zhang W. ref. 1b. :222–236.Zhang W. Curr Opin Drug Disc Dev. 2004;7:784.
- 10.(a) Luo ZY, Williams J, Read RW, Curran DP. J Org Chem. 2001;66:4261. doi: 10.1021/jo010111w. [DOI] [PubMed] [Google Scholar]; (b) Curran DP, Amatore M, Campbell M, Go E, Guthrie D, Luo Z. J Org Chem. 2003;68:4643. doi: 10.1021/jo0344283. [DOI] [PubMed] [Google Scholar]; (c) Cioffi CL, Berlin ML, Herr RJ. Synlett. 2003:841. [Google Scholar]; (d) Read R, Zhang C. Tetrahedron Lett. 2003;44:7045. [Google Scholar]; (e) Zhang W, Lu Y. Org Lett. 2003;5:2555. doi: 10.1021/ol034854a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Zhang W. Org Lett. 2003;5:1011. doi: 10.1021/ol027469e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Chen CHT, Zhang W. Org Lett. 2003;5:1015. doi: 10.1021/ol0274864. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Nagashima T, Zhang W. J Comb Chem. 2004;6:942. doi: 10.1021/cc049885r. [DOI] [PubMed] [Google Scholar]; (i) Zhang W, Chen CHT, Lu Y, Nagashima T. Org Lett. 2004;6:1473. doi: 10.1021/ol0496428. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Zhang W, Tempest P. Tetrahedron Lett. 2004;45:6757. [Google Scholar]; (k) Huang Y, Qing FL. Tetrahedron. 2004;65:8341. [Google Scholar]; (l) Villard AL, Warrington BH, Ladlow M. J Comb Chem. 2004;6:611. doi: 10.1021/cc0499338. [DOI] [PubMed] [Google Scholar]; (m) McAllister LA, McCormick RA, Brand S, Procter DJ. Angew Chem Int Ed. 2005;44:452. doi: 10.1002/anie.200461930. [DOI] [PubMed] [Google Scholar]
- 11.(a) de Visser PC, van Helden M, Filtppov DV, van der Marel GA, Drijfhout JW, van Boom JH, Noort D, Overkleeft HS. Tetrahedron Lett. 2003;44:9013. [Google Scholar]; (b) Beller C, Bannwarth W. Helv Chim Acta. 2005;88:171. [Google Scholar]; (c) Brittain SM, Ficarro SB, Brock A, Peters EC. Nature Biotech. 2005;23:463. doi: 10.1038/nbt1076. [DOI] [PubMed] [Google Scholar]
- 12.A 2x12 SPE manifold is available from Supelco, Fisher, and Waters.
- 13.For a special issue of SPE for sample preparations, see:Poole CF, Wilson ID. J Chrom A. 2000;885:1–471.
- 14.Argonaut VacMaster-96™ SPE manifold, product number 121–9600. Cartridges are placed on Isolute array-24 base plate, product number 121–9650 (www.argotech.com).
- 15.Wob-L® piston pump, model 2545 (www.welchvacuum.com).
- 16.Whatman® 24-well (10 mL) receiving plate, product number 7701–51022 (www.whatman.com).
- 17.FluoroFlash® silica gel cartridges, 3 g, 6 mL (www.fluorous.com).
- 18.Sudan blue II (17354-14-2) from Aldrich.
- 19.Markowicz MW, Dembinski R. Synthesis. 2004:80. [Google Scholar]
- 20.From Argonaut Technologies, Inc.
- 21.Nagashima T, Petro M, Lu Y, Zhang W. Tetrahedron Lett. in press. [Google Scholar]
- 22.After conducted coupling reactions with F-CDMT, we found literature which indicated that in DCM the complex of CDMT with NMM could cause NMM demethylation. The relatively low yields and purities listed in Table 3 may partially relate to using DCM as the reaction solvent. Reference for NMM demethylation, see: Kunishima M, Kawachi C, Iwasaki F, Terao K, Tani S. Tetrahedron Lett. 1999;40:5327.


