Abstract
The fluorous synthesis of disubstituted pyrimidines is carried out by attaching 2,4-dichloro-6-methylpyrimidine with 1H,1H,2H,2H-perfluorodecanethiol. The tagged substrate is substituted with 3-(trifluoromethyl)pyrazole followed by thioether oxidation and tag displacement with amines and thiols. The fluorous ponytail serves as a phase tag for intermediate and product purification over FluoroFlash™ SPE cartridges.
Because of their important biological activities, substituted N-heterocycles such as pyrimidines, quinazolines, and purines, are the targets of many solid-phase syntheses.1 The large majority of these reactions are based on the nucleophilic substitution of halogenated N-heterocycles attached to nitrogen- or sulfur-based linkers. The sulfur linker is popular because it can be activated by oxidation and displaced by a wide variety of nucleophiles to introduce new diversity to the molecule.2
Recently, fluorous technologies have been developed as solution-phase alternatives for high-throughput synthesis.3 Functionalized perfluoroalkyl groups, instead of polymers, are used as phase tags attached to substrates or reagents for fluorous synthesis.4 Fluorous mixture synthesis5 and parallel synthesis using fluorous reagents6, scavengers7, and protecting groups5,8 have previously been reported. Described in this paper is a fluorous catch and release technique for the synthesis of disubstituted pyrimidines.
Fluorous thiols are good nucleophiles that have been used as scavengers for halides in parallel synthesis of a secondary amine library.7a To demonstrate its utility as a phase tag in fluorous synthesis, a fluorous thiol 1H,1H,2H,2H-perfluorodecanethiol was attached to the electron deficient 2,4-dichloro-6-methylpyrimidine by a nucleophilic substitution (Scheme 1).
Scheme 1.
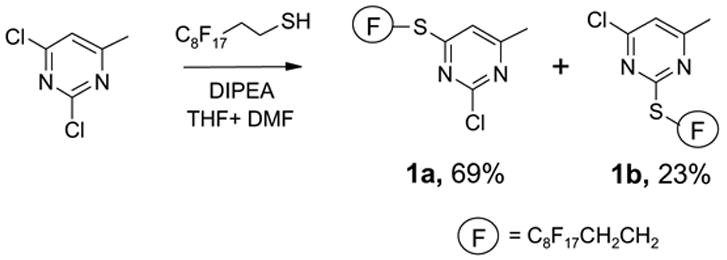
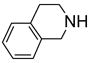
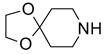
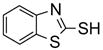
Two regioisomers 1a and 1b were generated in a ratio of 3:1 by HPLC analysis. If polymeric tags were used, regioisomers like 1a and 1b would not be separated. However, fluorous compounds 1a and 1b were readily separated by flash column chromatography on normal silica gel based on their different polarity. The major isomer 1a was used for further nucleophilic substitution with 3-(trifluoromethyl)pyrazole to give 2 (Scheme 2). The fluorous sulfur tag was then activated by oxidation with Oxone™ followed by the displacement with ten assorted nucleophiles including primary and secondary amines and thiols to yield disubstituted pyrimidines 4 (Table 1).
Scheme 2.
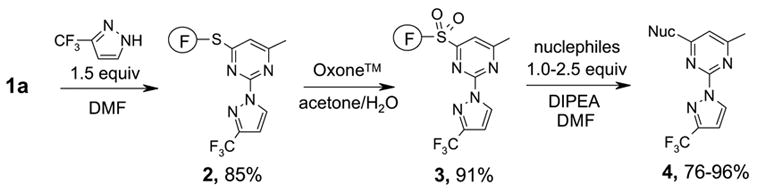
Table 1.
Structures, yields, and purities of disubstituted pyrimidines
| nucleophile | equiv | yield (purity)a | nucleophile | equiv | yield (purity) | |||
|---|---|---|---|---|---|---|---|---|
|
a |
|
2.0 | 96% (97%) | f |
|
1.5 | 88% (89%) |
| b |
|
2.0 | 91% (93%) | g |
|
1.5 | 74% (93%) | |
| c |
|
2.0 | 82% (92%) | h |
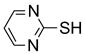
|
1.0 | 76% (97%) | |
| d |
|
2.5 | 93% (90%) | i |
|
1.0 | 84% (92%) | |
| e |
|
2.5 | 79% (90%) | j |
|
1.0 | 77% (90%) |
purity was assessed by 1H NMR and HPLC on a C18 column with a UV 254nm detector
An important feature of fluorous synthesis is the employment of simple solid-phase extraction (SPE) over FluoroFlash™ cartridges charged with fluorous silica.4,9 Only two fractions need to be collected from the SPE; a MeOH/H2O (80/20) fraction containing non-fluorous compounds and a MeOH fraction containing fluorous compounds. The strong fluorine-fluorine interaction retains small molecules tagged with a light fluorous ponytail (C8F17) on fluorous silica gel until elution with a stronger solvent such as MeOH. Fluorous intermediates 2 and 3 were purified by SPE and collected in the MeOH fraction, while product 4 was collected in the MeOH/H2O fraction. Because diisopropylethylamine (DIPEA) and excess nucleophiles were used for the last reaction step, a small amount of weak acidic ion exchange resin (Amberlite™ CG-50) was placed on top of the fluorous silica for the retention of both the cleaved fluorous tag and amines.7c We found that CF3 group of products 4 did not hold the molecules against elution with MeOH/H2O. All products had purities greater than 90% after SPE. The 1H NMR spectra of intermediate 2 and product 4e demonstrate the efficiency of SPE (Figure 1). The crude intermediate 2 was separated from the excess 3-(trifluoromethyl)pyrazole by collecting the MeOH fraction. The crude product 4e containing excess 4-phenylpiperazine, DIPEA, and cleaved fluorous tag was purified by collecting the MeOH/H2O fraction from a cartridge charged with fluorous silica and acidic ion exchange resin.
Figure 1.
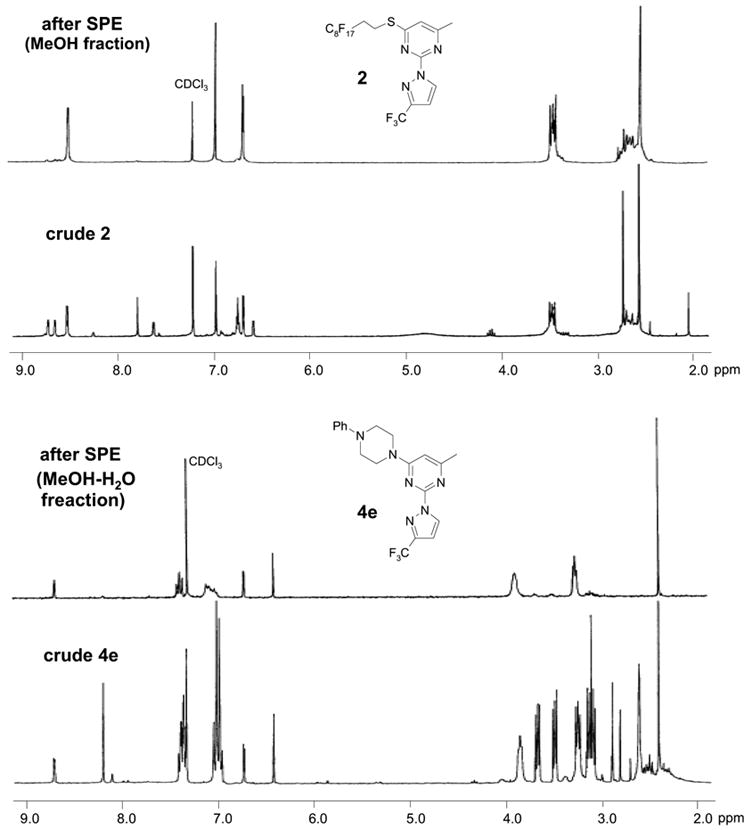
1H NMR spectra of intermediate 2 and product 4e before and after SPE
Synthesis of substituted pyrimidines exemplifies the unique characters of fluorous synthesis, including the use of tag strategy for quick SPE, analysis and separation of tagged-isomers by conventional tools, and adaptability of traditional solution-phase reaction conditions. The “beadless” and traceless fluorous thiol tag is complementary and supplementary to corresponding thiol linkers in solid-phase synthesis. The catch and release method with the fluorous thiol has good potential in the synthesis of other substituted N-heterocyles.
Acknowledgments
We thank the National Institutes of General Medical Sciences SBIR funding (2 R44 GM062717-02).
References and notes
- 1.a) Ding S, Gray NS, Wu X, Ding Q, Schultz G. J Am Chem Soc. 2002;124:1594. doi: 10.1021/ja0170302. [DOI] [PubMed] [Google Scholar]; b) Ding S, Gray NS, Wu X, Ding Q, Schultz G. J Comb Chem. 2002;4:183. doi: 10.1021/cc010080i. [DOI] [PubMed] [Google Scholar]; c) Luo G, Chen L, Poindexter GS. Tetrahedron Lett. 2002;43:5739. [Google Scholar]; d) Dener JM, Lease TG, Novack AR, Plunkett MJ, Hocker MD, Fantauzzi PP. J Comb Chem. 2001;3:590. doi: 10.1021/cc010027u. [DOI] [PubMed] [Google Scholar]; e) Lucrezia RD, Gilbert IH, Floyd CD. J Comb Chem. 2000;3:249. doi: 10.1021/cc990063h. [DOI] [PubMed] [Google Scholar]; f) Parrot I, Wermuth CG, Hibert M. Tetrahedron Lett. 1999;40:7975. [Google Scholar]
- 2.a) Brun V, Legraverend M, Grieson DS. Tetrahedron. 2002;58:7911. [Google Scholar]; b) Ding S, Gray NS, Wu X, Ding Q, Schultz G. J Org Chem. 2001;66:8273. doi: 10.1021/jo016010f. [DOI] [PubMed] [Google Scholar]; c) Gayo LM, Suto MJ. Tetrahedron Lett. 1997;38:211. [Google Scholar]
- 3.a) Curran DP. Chemtracts-Org Chem. 1996;9:75. [Google Scholar]; b) Curran DP. Angew Chem Int Ed Eng. 1998;37:1175. [Google Scholar]; f) Curran DP. In: Stimulating Concepts in Chemistry. Stoddard F, Reinhoudt D, Shibasaki M, editors. Wiley-VCH; New York: 2000. p. 25. [Google Scholar]; d) Curran DP, Hadida S, Studer A, He M, Kim S-Y, Luo Z, Larhed M, Hallberg M, Linclau B. In: Combinatorial Chemistry: A Practical Approach. Fenniri H, editor. Vol. 2. Oxford University Press; Oxford: 2001. p. 327. [Google Scholar]; e) Gladysz JA, Curran DP. Tetrahedron. 2002;58:3823. [Google Scholar]
- 4.Curran DP. Synlett. 2001:1488. [Google Scholar]
- 5.a) Luo ZY, Zhang QS, Oderaotoshi Y, Curran DP. Science. 2001;291:1766. doi: 10.1126/science.1057567. [DOI] [PubMed] [Google Scholar]; b) Zhang W, Zhiyong Luo, Christine Chen, Dennis P Curran.J Am Chem Soc 20021241044312197746 [Google Scholar]; c) Zhang Q, Rivkin A, Curran DP. J Am Chem Soc. 2002;124:5774. doi: 10.1021/ja025606x. [DOI] [PubMed] [Google Scholar]; d) Curran DP, Furukawa T. Org Lett. 2002;4:2233. doi: 10.1021/ol026084t. [DOI] [PubMed] [Google Scholar]
- 6.a) Dandapani S, Curran DP. 2002;58:3855. [Google Scholar]; b) Crich D, Neelamkavil S. Tetrahedron. 2002;58:3865. [Google Scholar]
- 7.a) Zhang W, Curran D, Chen CHT. Tetrahedron. 2002;58:3871. [Google Scholar]; b) Lindsley CW, Zhao Z, Leister WH. Tetrahedron Lett. 2002;43:4225. [Google Scholar]; c) Lindsley CW, Zhao Z, Leister WH, Strauss KA. Tetrahedron Lett. 2002;43:6319. [Google Scholar]
- 8.a) Wipf P, Reeves JT. Tetrahedron Lett. 1999;40:4649. [Google Scholar]; b) Rover S, Wipf P. Tetrahedron Lett. 1999;40:5667. [Google Scholar]; b) Wipf P, Reeves J. Tetrahedron Lett. 1999;40:5139. [Google Scholar]; c) Luo ZY, Williams J, Read RW, Curran J Org Chem. 2001;66:4261. doi: 10.1021/jo010111w. [DOI] [PubMed] [Google Scholar]
- 9.Fluorous thiol and FluoroFlash™ SPE cartridges are available from Fluorous Technologies, Inc. www.fluorous.com


