Abstract
Background
Factors that influence energy metabolism and substrate oxidation, such as thyroid hormones (THs), may be important regulators of body weight.
Objective
We investigated associations of THs cross-sectionally with obesity, energy expenditure, and substrate oxidation and prospectively with weight change.
Design
Euthyroid, nondiabetic, healthy, adult Pima Indians (n = 89; 47 M, 42 F) were studied. Percentage body fat (%BF) was measured by using dual-energy X-ray absorptiometry; sleeping metabolic rate (SMR), respiratory quotient, and substrate oxidation rates were measured in a respiratory chamber. Thyroid-stimulating hormone (TSH), free thyroxine (T4), free triiodothyronine (T3), and leptin concentrations were measured in fasting plasma samples.
Results
TSH, but neither free T3 nor free T4, was associated with %BF and leptin concentrations (r = 0.27 and 0.29, respectively; both: P ≤ 0.01). In multiple regression analyses adjusted for age, sex, fat mass, and fat-free mass, free T3 was a positive predictor of SMR (P = 0.02). After adjustment for age, sex, %BF, and energy balance, free T3 was a negative predictor of 24-h respiratory quotient (P < 0.05) and a positive predictor of 24-h lipid oxidation rate (P = 0.006). Prospectively, after an average follow-up of 4 ± 2 y, the mean increase in weight was 3 ± 9 kg. Baseline T3 concentrations were associated with absolute and annual percentage of changes in weight (r = −0.27, P = 0.02, and r = −0.28, P = 0.009, for the age-and sex-adjusted associations, respectively).
Conclusions
In euthyroid Pima Indians, lower free T3 but not free T4 concentrations were an independent predictor of SMR and lipid oxidation and a predictor of weight gain. This finding indicates that control of T4-to-T3 conversion may play a role in body weight regulation.
Keywords: Thyroid hormones, energy expenditure, lipid oxidation, obesity, respiratory chamber
INTRODUCTION
Obesity is a disease of energy imbalance. Lower 24-h energy expenditure (EE) (1), lower resting metabolic rate (1), and higher respiratory quotient (RQ), which indicates a lower whole-body lipid oxidation (LOX) rate (2, 3), are all predictors of weight gain. Even after adjustment for the main predictors of these metabolic variables (ie, body composition, body size, fat distribution, age, sex, familiar aggregation, and glucose tolerance status), a significant proportion of the variance in 24-h EE (4, 5), resting metabolic rate (6), and sleeping EE (4, 5) remains unexplained. This unexplained variance is even larger for RQ (3, 5). For the prevention or treatment of obesity or both, therefore, it is important to identify additional physiologic mechanisms underlying the variability in EE and substrate oxidation.
Thyroid hormones (THs) stimulate thermogenesis by increasing ATP consumption during TH-dependent processes (ie, substrate cycles, maintenance of ion gradients, and Ca2+ transfer from cytosol to sarcoplasmic reticulum) and decreasing the efficiency of ATP synthesis (7). For the latter mechanism, transcriptional control of several genes, including uncoupling proteins (8) and mitochondrial glycerol-3-phosphate dehydrogenase (9), may mediate a significant fraction of TH-induced thermogenesis. THs influence lipid metabolism (10) by increasing sensitivity tosympatheticnervoussystem-mediatedlipolysis(11–13). Further-more, THs can also increase the oxidation of fatty acids by stimulating the expression of carnitine palmitoyl transferase (14).
Evidence for a significant role of THs in energy metabolism and substrate cycles comes mainly from observations made during experimental or spontaneous forms of thyroid dysfunction, which are associated with changes in energy intake, body weight and composition, macronutrient balances, body temperature, and EE. However, whether subtle differences in thyroid function within the normal range influence body weight, energy metabolism, and substrate oxidation is unclear. We explored the cross-sectional relations of plasma concentrations of thyroid-stimulating hormone (TSH) and THs with the above-mentioned variables in euthyroid, nondiabetic, adult Pima Indians and examined the relation of THs with weight change in these persons.
SUBJECTS AND METHODS
Subjects
The Gila River (Pima) Indian Community participates in an ongoing longitudinal study to identify risk factors for obesity and type 2 diabetes. We selected healthy nondiabetic adults who were at least three-quarters Pima or were closely related Tohono O’odham and who were euthyroid, according to normal serum TSH concentrations at admission to the clinical research unit (CRU) and retrieved from computerized laboratory records since 1997, who had measurements of energy metabolism and substrate oxidation in a respiratory chamber, and who had a nondiabetic follow-up visit with recorded weight. At this last visit, they were taking no medications that could affect weight (including antithyroidals or levothyroxine) or had a diagnosis of hypothyroidism or hyperthyroidism on review of medical records. At baseline, all subjects were admitted to the National Institutes of Health CRU of the National Institute of Diabetes and Digestive and Kidney Diseases in Phoenix, AZ, where they were fed a weight-maintaining diet (50% of calories from carbohydrate, 30% from fat, and 20% from protein) throughout their stay on the metabolic ward. Procedures were performed after ≥3 d on this diet. Subjects did not smoke or take medications at the time of the study.
All subjects gave written informed consent. The study was approved by the National Institute of Diabetes and Digestive and Kidney Diseases Institutional Review Board and the Gila River Indian Tribal Council.
Methods
Height was measured with the use of a stadiometer; weight was obtained with an electronic digital scale. Percentage body fat (%BF) was measured by using dual-energy X-ray absorptiometry. Glucose tolerance status was assessed by using a 75-g oral-glucose-tolerance test (15). EE and substrate oxidation were assessed in a respiratory chamber (6). In brief, volunteers entered the chamber at 0745 after an overnight fast and remained therein for 23 h. Meals were provided at 0800, 1130, and 1700, and an evening snack was provided at 2000. The rate of EE was measured continuously, calculated for each 15-min interval and then averaged for the 24-h interval (24-h EE). Sleeping metabolic rate (SMR) was defined as the average EE of all 15-min periods between 2330 and 0500 during which spontaneous physical activity (assessed by a motion radar) was <1.5%. Carbon dioxide production and oxygen consumption were calculated at 15-min intervals for the 23 h in the chamber and then extrapolated to 24 h. The 24-h RQ was calculated as the ratio of 24-h carbon dioxide production to 24-h oxygen consumption. Acute energy balance (ENBAL) (ie, 24-h ENBAL during the stay in the respiratory chamber) was calculated as 24-h energy intake minus 24-h EE. On the basis of 24-h EE, 24-h RQ, and 24-h urinary nitrogen excretion, the rates for 24-h carbohydrate, lipid, and protein oxidation were calculated as previously described (16). Carbohydrate, fat, and protein balances were calculated as 24-h substrate intake minus 24-h substrate oxidation.
Laboratory analyses
Serum TSH at the admission to the CRU was measured at the Phoenix Indian Medical Center laboratory by using a colorimetric immunoassay (Dade Behring, Newark, DE), with intraassay and interassay CVs of 3.1% and 5%, respectively. However, frozen fasting plasma samples, collected after ≥3 d on a weight-maintaining diet, were used to measure TSH, free TH, and leptin concentrations at Linco-Diagnostic Services, Inc (St Charles, MO). Plasma TSH and leptin were measured by radioimmunoassay (LincoPlex; Linco-Diagnostic Services, Inc) with intraassay and interassay CVs of 6.9% and 3% and 5.1% and 7.4%, respectively. Plasma free triiodothyrionine (T3) and free thyroxine (T4) concentrations were measured by using a solid-phase 125I radioimmunoassay (Diagnostic Product Corporation, Los Angeles, CA) with intraassay and interassay CVs of 6% and 7.8% and 5% and 7%, respectively.
Statistical analyses
Cross-sectional analysis
Unadjusted sex differences in plasma hormone concentrations were evaluated by Student’s t tests and nonparametric (Kruskall-Wallis) test. Spearman’s correlation analysis was used to quantify cross-sectional relations among plasma hormone concentrations, anthropometric variables, and metabolic variables. General linear regression models were used to assess independent relations of THs with 24-h EE and SMR, with both adjusted for age, sex, fat mass, and fat-free mass (FFM) (5). General linear regression models were also used to investigate the association of THs with 24-h RQ and oxidation of lipids, carbohydrates, and proteins, with all adjusted for age, sex, ENBAL, and %BF (5). Stepwise regression analysis was used afterward to estimate the individual contribution (partial R2) of THs to the variance of the dependent variables.
Prospective analysis
Spearman’s correlation analysis was used to quantify associations between THs at baseline and changes in weight. The associations were expressed as absolute changes in weight (in kg) (final weight – initial weight) or the percentage total weight change per year ({[(final weight – initial weight)/initial weight]/y of follow-up} × 100).
Statistical analyses were performed by using SAS software (version 9; SAS Institute Inc, Cary, NC). Data are expressed as means ± SDs throughout. Nonnormally distributed variables were log transformed (log10) before statistical analysis to approximate a normal distribution. Significance was P < 0.05.
RESULTS
Subject characteristics are shown in Table 1. Neither plasma TSH nor free T3 concentrations differed significantly by sex (P > 0.3 for both). Free T4 was significantly higher in men (P = 0.009) and leptin was significantly higher in women (P < 0.0001). After adjustment for age and %BF, free T4 concentrations did not differ significantly between the sexes (P = 0.06), and leptin concentrations remained significantly higher in women (P = 0.002). Although all subjects had a normal serum TSH concentration on admission (range: 0.6–4.6 μIU/mL), a few persons (n = 10) had plasma TSH concentrations from samples drawn several days later that were above the cutoff for normal serum values (normal ranges for plasma TSH were not available) (Figure 1).
TABLE 1.
Subject characteristics1
| Characteristics | Value |
|---|---|
| Sex | |
| Females (n) | 42 |
| Males (n) | 47 |
| Glucose tolerance | |
| Normal (n) | 60 |
| Impaired (n) | 29 |
| Age (y) | 29 ± 7 (18–44)2 |
| Height (cm) | 167 ± 7 (152–183) |
| Body weight (kg) | 93 ± 20 (55–137) |
| Percentage body fat (%) | 33 ± 7 (14–46) |
| Fat-free mass (kg) | 61 ± 11 (41–85) |
| Fasting glucose (mg/dL) | 87 ± 9 (64–116) |
| 2-h OGTT (mg/dL) | 121 ± 30 (51–197) |
| Fasting insulin (μU/mL) | 44 ± 18 (13–112) |
| TSH (μIU/mL) | 2.75 ± 1.69 (0.35–8.94) |
| Free T3 (pg/mL) | 2 ± 0.4 (1–2.94) |
| Free T4 (ng/dL) | |
| Males | 1.3 ± 0.3 (0.78–1.97) |
| Females | 1.1 ± 0.2 (0.71–1.55) |
| Leptin (ng/mL) | |
| Males | 9.6 ± 4.9 (1.8–21.2) |
| Females | 28.9 ± 10.6 (4.4–45.7) |
| 24-h Energy expenditure (kcal/d) | 2340 ± 385 (1545–3332) |
| Sleeping metabolic rate (kcal/d)3 | 1642 ± 230 (1164–2164) |
| 24-h Respiratory quotient | 0.85 ± 0.02 (0.8–0.9) |
| 24-h Carbohydrate oxidation (kcal/d) | 1113 ± 219 (549–1677) |
| 24-h Lipid oxidation (kcal/d) | 936 ± 267 (431–1729) |
| 24-h Energy balance (kcal/d) | −162 ± 222 (−745 to 286) |
| 24-h Carbohydrate balance (kcal/d) | −23 ± 163 (−414 to 503) |
| 24-h Fat balance (kcal/d) | −282 ± 236 (−1069 to 149) |
| 24-h Protein balance (kcal/d) | 164 ± 99 (−58 to 475) |
n = 89. TSH, thyroid-stimulating hormone; OGTT, oral-glucose-tolerance test; T3, triiodothyronine; T4, thyroxine. Insulin (μUI/mL), glucose (mg/dL), TSH (μIU/mL), free T3 (pg/mL), and free T4 (ng/dL) concentrations are given in conventional units; to convert to SI units (pmol/L, mmol/L, μIU/L, pmol/L, and pmol/L, respectively), multiply by 6.945, 0.055, 1, 1.54, and 12.87, respectively.
x̄ ± SD; range in parentheses (all such values).
Data available for 86 subjects.
FIGURE 1.
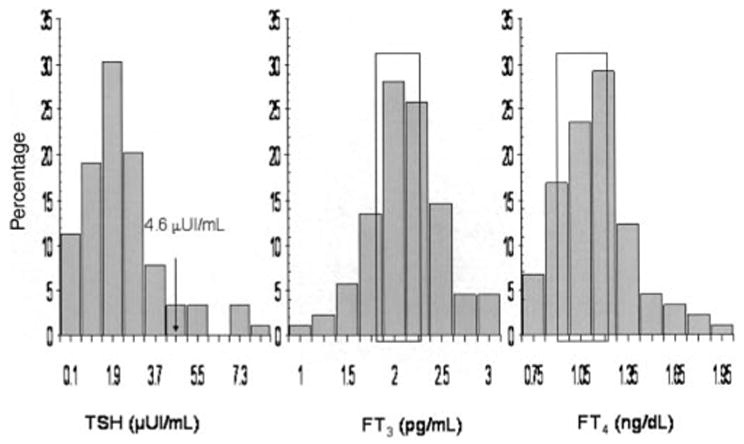
Distributions of plasma concentrations of thyroid-stimulating hormone (TSH), free triiodothyronine (FT3), and free thyroxine (FT4) in 89 persons. FT3 and FT4 concentrations in subjects with plasma TSH concentrations above the normal range for serum TSH (ie, 4.6 μUI/mL; n = 10) were within the limits indicated in the figure (boxes) and above the 25th and 10th percentiles of the study population, respectively.
Sex-adjusted free T3 and free T4 plasma concentrations were not associated with adiposity. Plasma TSH concentrations were positively associated with body weight and %BF (r = 0.31 and r = 0.27, respectively; both: P ≤ 0.01). Plasma TSH and leptin concentrations were positively associated (r = 0.29, P = 0.006), but this association was no longer significant after adjustment for sex and %BF (partial r = 0.15, P = 0.15). Neither TSH nor leptin concentrations were associated with free T3 or free T4 (P > 0.5). Plasma concentrations of free T3 and free T4 were positively correlated (r = 0.47, P < 0.0001).
Thyroid hormones, energy metabolism, and substrate oxidation
No relation was found between free T3 and 24-h EE. However, in a multiple regression analysis with age, sex, fat mass, and FFM as covariates, free T3 was an independent predictor of SMR (Table 2). Free T3 contributed 1.2% of the total variance of SMR explained by this model. Age (P = 0.03) and FFM (P < 0.001) were additional predictors of SMR. The ratio of free T3 to free T4 (T3:T4) was also an independent predictor of SMR and explained 1.8% (P < 0.005) of its total variance. In regression models adjusted for age, sex, %BF, and ENBAL, free T3 was an independent predictor of 24-h RQ (Table 2), contributing to 4% of its total variance. ENBAL (P < 0.001) was also a predictor of 24-RQ in this model. Free T3 was positively and significantly associated with 24-h LOX but not with 24-h carbohydrate oxidation rate (P = 0.004 and P = 0.4, respectively). In multiple regression analysis, free T3 remained associated with 24-h LOX independently of age, sex, %BF, and ENBAL (Table 2), contributing 6% of the variance explained by this model. Sex, %BF, and ENBAL (all: P < 0.001) were additional predictors of 24-h LOX. No significant relation was found between either TSH or free T4 plasma concentration and energy metabolism or substrate oxidation.
TABLE 2.
Adjusted association between free triiodothyronine (T3) concentrations and metabolic variables by general linear regression analysis in 89 subjects1
| SMR2 |
24-h RQ3 |
24-h LOX4 |
Fat balance5 |
|||||
|---|---|---|---|---|---|---|---|---|
| β | P | β | P | β | P | β | P | |
| Free T3 | 74.4 | 0.02 | −0.01 | <0.05 | 126 | 0.006 | −132 | 0.004 |
Data are β (estimated partial regression coefficient) and P values for the association of free T3 concentrations (x̄ ± SD: 2 ± 0.4 pg/mL) with metabolic variables after adjustment for covariates. SMR, sleeping metabolic rate; RQ, respiratory quotient; LOX, lipid oxidation.
Data available in 86 subjects. SMR (1642 ± 230 kcal/d) adjusted for age, sex, fat mass, and fat-free mass. Total R2 = 0.83 and P < 0.0001 for the entire model.
RQ (0.85 ± 0.02) adjusted for age, sex, percentage body fat, and energy balance. Total R2 = 0.28 and P < 0.0001 for the entire model.
LOX (936 ± 267 kcal/d) adjusted for age, sex, percentage body fat, and energy balance. Total R2 = 0.49 and P < 0.0001 for the entire model.
Fat balance (−282 ± 236 kcal/d) adjusted for age, sex, percentage body fat, and energy balance. Total R2 = 0.55 and P < 0.0001 for the entire model.
Total acute energy balance, substrate balances, and thyroid hormones
ENBAL was strongly correlated with fat balance (sex-partial r = 0.69, P < 0.0001) and weakly correlated with carbohydrate (sex-partial r = 0.20, P = 0.06) and protein balances (sex-partial r = 0.23, P = 0.03). A significant y intercept was observed for the sex-adjusted regression line of ENBAL compared with fat and protein balances (−177 ± 30 and 140 ± 13 kcal/d, respectively; both: P < 0.0001). The subjects thus tended to be in a negative fat balance and a positive protein balance when ENBAL was zero. Free T3 was associated with fat (sex-partial r = −0.27, P = 0.01), carbohydrate (sex-partial r = 0.22, P = 0.04), and protein (sex-partial r = 0.19, P = 0.07) balances. However, in multiple regression analysis adjusted for age, sex, ENBAL, and %BF, free T3 was an independent predictor of fat (Table 2) but not protein or carbohydrate balances (β = 31 and β = 65 respectively; both: P = 0.15).
Prospective analysis
Average follow-up was 4 ± 2 y. During this time, the mean increase in weight was 3 ± 9 kg. Neither absolute nor annual percentage of changes in weight were associated with initial weight (both P = 0.9). Baseline T3 concentrations were associated with absolute (r = −0.23, P = 0.03) and annual (Figure 2) percentage changes in weight even after adjustment for baseline age and sex (r = −0.27, P = 0.02, and r = −0.28, P = 0.009, respectively). T3:T4 also showed negative but borderline (P ≤ 0.1) associations with weight change.
FIGURE 2.
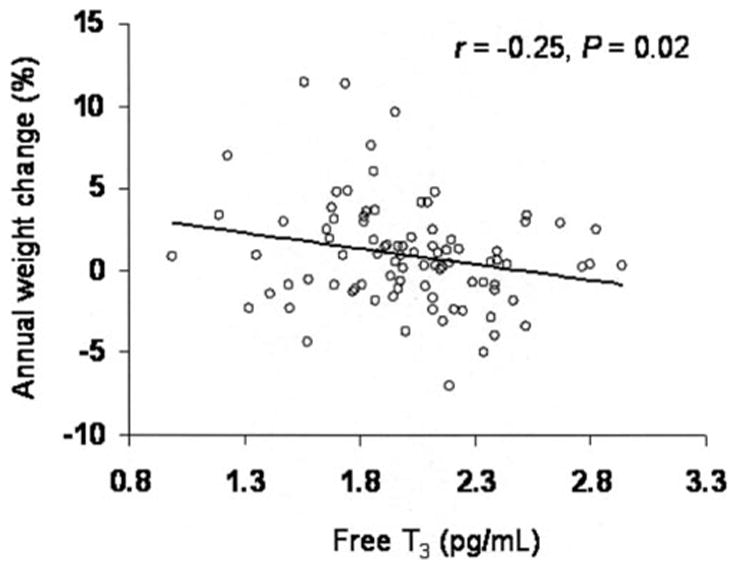
Relation between baseline free triiodothyronine (T3) concentrations and weight change in 89 persons. Spearman’s simple correlation coefficient and P value for the association of free T3 (x̄ ± SD: 2 ± 0.4 pg/mL) and annual percentage of weight change (x̄ ± SD: 1 ± 3%) are given.
DISCUSSION
Lower concentrations of free T3 predicted weight gain in euthyroid nondiabetic Pima Indians. In cross-sectional analysis, free T3 but not free T4 concentrations were independently associated with both SMR and LOX rate, whereas only TSH concentrations (ie, not free T4 or free T3) were associated with adiposity.
In patients undergoing chronic treatment with thyroxine, small changes in the daily dose, which still maintained free T4 concentrations within the normal range, were associated with changes in resting EE (17). Moreover, studies in euthyroid subjects have found that serum or plasma T3 concentrations were an independent predictor of 24-h EE (4), SMR (4, 18), and resting metabolic rate (19). SMR, which is an accurate estimate of basal metabolic rate, is the largest component of 24-h EE and is not influenced by the thermic effect of food or the energy cost of physical activity. In the current study, free T3 and free T3:T4 were associated with SMR, and, as in previous studies (4, 18, 19), they contributed (1–2%) to its variance independently of FFM. Although small, this contribution represents 5–10% (6.3% in this study) of the variance in SMR unexplained by body size, which accounts for 80–85% (81% in this study) of the EE variance (5).
In agreement with a previous report (20), 24-h ENBAL was strongly correlated only with net fat balance, which indicated that even a small degree of overfeeding on a mixed diet would result mostly in fat storage. Indeed, a lower LOX rate, which predisposes to a positive fat balance, is a predictor of weight gain (2, 3). The association found between free T3 and 24-h RQ, a ratio of carbohydrate oxidation rate to LOX rate, has not been reported previously in euthyroid persons. This negative relation was explained by the positive and independent association of free T3 with LOX rate and the lack of association with carbohydrate oxidation. Because lower EE is also a risk factor for weight gain (1), it is not surprising that free T3, which was a predictor of both SMR and LOX rate, predicted changes in body weight in this population.
In this study, TSH but not free T3 or free T4 was associated with adiposity. Free TH concentrations, which are unaffected by factors modifying serum-binding proteins, were reported to be positively (21), negatively (22–25), or not (21, 22, 26–28) associated with adiposity. Two large cross-sectional studies showed a positive association between circulating TSH and adiposity (24, 29). Spontaneous 24-h TSH secretion was also enhanced in obese compared with lean women (27). Some animal (30, 31) and human (27, 32) studies indicate that leptin may mediate this association. The increased activity of the hypothalamic-pituitary-thyroid axis in response to increasing leptin concentrations could act as a check against further weight gain, in part through the metabolic effects of THs. However, leptin administration failed to increase TSH concentrations in both healthy adults (33) and children with congenital leptin deficiency (34). In this study, we observed no association between leptin and TSH after adjustment for adiposity, and no association was observed between adiposity, TSH, and leptin or free TH concentration, as would have been expected under the above hypothesis. Therefore, mechanisms other than leptin may contribute to increased TSH concentrations in obese people.
In this study, free T3 but not free T4 concentrations were associated with metabolic variables and predicted weight change. T4, the main product of thyroid secretion, must be activated by deiodination to the biologically active hormone T3 by type 1 deiodinase (D1) or type 2 deiodinase (D2). Free T3:T4 is considered an estimate of deiodination activity. D2 is a selenoenzyme mainly expressed in the central nervous system, pituitary and thyroid glands, skeletal muscle, and adipose tissue (35). A complex control of D2 activity is critical for the T4-mediated negative feedback in thyrotrophic cells (36). D2 is also a main determinant of both nuclear TH receptor-bound T3 (37) and plasma T3 concentrations in euthyroid humans (38). Therefore, a decrease in D2 activity in metabolically active tissues (such as adipose or muscle tissue) could explain the observed association of free T3 concentrations with SMR, LOX, and weight change. This decrease in central D2 activity may affect the feedback mechanism so that a slight decrease in intracellular T3 would promote an increase in TSH secretion, and that may explain the lack of association between TSH and free T4 concentrations in our subjects. Factors modifying D2 activity beside T4 concentrations (38) have not been clearly identified. However, genetic polymorphisms of the D2 gene, which were not associated with TH concentrations in this study (data not shown; 39, 40), deficiency in selenium (41) or alterations in its incorporation into D2 (42), oxidative stress (43), inflammation (44), and nutritional factors (45–48), can modify D2 activity. Although D2 may play an important role in these associations, D1, which is both an activating and deactivating enzyme and which contributes to serum T3 concentrations (38, 49), and D3 (an inactivating enzyme) may also play a significant role in these associations.
On the basis of the cross-sectional associations, we have speculated about a possible effect of lower free T3 concentrations on weight gain through a decrease in resting EE and LOX rate. However, SMR and 24-h LOX were not predictors of weight change in this population (data not shown), and adjustment for these variables did not modify the association of baseline free T3 concentrations and weight change. This finding could be due to differences in the ability to measure SMR and 24-h LOX (greater interindividual variability or less accuracy) compared with free T3 concentrations, or it may indicate that other mechanisms, including energy intake and levels of physical activity and its energy cost, mediate the association of free T3 with body weight gain. Furthermore, sympathetic nervous system activity is a confounder not accounted for in this study. Sympathetic nervous system activity predicts weight change (50), stimulates the peripheral conversion of T4 to T3 (51–53), and inhibits food intake (54). THs are involved in multiple processes; therefore, the effects of TH on weight gain may occur by the additive effect of several mechanisms rather than by a modulation of a single fundamental process.
A normal serum TSH concentration, measured on the day of the admission, was our inclusion criterion to study only euthyroid persons. However, plasma TSH concentrations, measured ≥3 d after stabilization with a weight-maintaining diet, were found to be slightly elevated in some of the subjects (n = 10), according to the upper cutoff established for serum TSH concentrations. Differences in the assay, type of sample used (plasma compared with serum), intraindividual variability over time, and time of venipuncture are factors that could explain this discrepancy (55). Subjects with this mild elevation in plasma TSH, however, had normal free T4 and free T3 concentrations. When they were excluded from the analysis, results did not change (data not shown).
Although our data show that low T3 concentrations are associated with weight gain, it is important to acknowledge that, because of its cardiovascular and protein-wasting effects, TH treatment is not a safe strategy in a pharmacologic approach to the problem of obesity. Whether tissue-selective (specifically, muscle) modifications in deiodinase activity may be a suitable target for drug intervention remains unclear (56). It is intriguing, however, that supplementation of a high-fat diet with bile acids increased thermogenesis by the selective activation of D2 in brown adipose tissue (the thermogenic organ in mice) and that it protected these mice from the obesigenic effect of this diet (48).
In summary, free T3 but not free T4 plasma concentrations are associated with SMR, LOX rate, and weight change in euthyroid adult persons. This finding indicates that factors influencing the conversion of T4 to T3 could play a role in energy homeostasis and body weight regulation.
Acknowledgments
We thank Joy C Bunt for critical reading of the manuscript; the clinical, dietary, and laboratory staffs of the NIDDK Clinical Research Unit; and especially Tom Anderson for his technical assistance. Most of all, we thank the volunteers for their participation in this study and the leaders of the Gila River Indian Community for their continuing support of our research.
Footnotes
Supported by the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases.
References
- 1.Ravussin E, Lillioja S, Knowler WC, et al. Reduced rate of energy expenditure as a risk factor for body-weight gain. N Engl J Med. 1988;318:467–72. doi: 10.1056/NEJM198802253180802. [DOI] [PubMed] [Google Scholar]
- 2.Seidell JC, Muller DC, Sorkin JD, Andres R. Fasting respiratory exchange ratio and resting metabolic rate as predictors of weight gain: the Baltimore Longitudinal Study on Aging. Int J Obes Relat Metab Disord. 1992;16:667–74. [PubMed] [Google Scholar]
- 3.Zurlo F, Lillioja S, Esposito-Del Puente A, et al. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: study of 24-h RQ. Am J Physiol. 1990;259:E650–7. doi: 10.1152/ajpendo.1990.259.5.E650. [DOI] [PubMed] [Google Scholar]
- 4.Toubro S, Sorensen TI, Ronn B, Christensen NJ, Astrup A. Twenty-four-hour energy expenditure: the role of body composition, thyroid status, sympathetic activity, and family membership. J Clin Endocrinol Metab. 1996;81:2670–4. doi: 10.1210/jcem.81.7.8675595. [DOI] [PubMed] [Google Scholar]
- 5.Weyer C, Snitker S, Rising R, Bogardus C, Ravussin E. Determinants of energy expenditure and fuel utilization in man: effects of body composition, age, sex, ethnicity and glucose tolerance in 916 subjects. Int J Obes Relat Metab Disord. 1999;23:715–22. doi: 10.1038/sj.ijo.0800910. [DOI] [PubMed] [Google Scholar]
- 6.Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest. 1986;78:1568–78. doi: 10.1172/JCI112749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silva JE. Thermogenic mechanisms and their hormonal regulation. Physiol Rev. 2006;86:435–64. doi: 10.1152/physrev.00009.2005. [DOI] [PubMed] [Google Scholar]
- 8.Lanni A, Moreno M, Lombardi A, Goglia F. Thyroid hormone and uncoupling proteins. FEBS Lett. 2003;543:5–10. doi: 10.1016/s0014-5793(03)00320-x. [DOI] [PubMed] [Google Scholar]
- 9.Lee YP, Lardy HA. Influence of thyroid hormones on L-alpha-glycerophosphate dehydrogenases and other dehydrogenases in various organs of the rat. J Biol Chem. 1965;240:1427–36. [PubMed] [Google Scholar]
- 10.Freake HC, Oppenheimer JH. Thermogenesis and thyroid function. Annu Rev Nutr. 1995;15:263–91. doi: 10.1146/annurev.nu.15.070195.001403. [DOI] [PubMed] [Google Scholar]
- 11.Haluzik M, Nedvidkova J, Bartak V, et al. Effects of hypo- and hyperthyroidism on noradrenergic activity and glycerol concentrations in human subcutaneous abdominal adipose tissue assessed with microdialysis. J Clin Endocrinol Metab. 2003;88:5605–8. doi: 10.1210/jc.2003-030576. [DOI] [PubMed] [Google Scholar]
- 12.Rubio A, Raasmaja A, Maia AL, Kim KR, Silva JE. Effects of thyroid hormone on norepinephrine signaling in brown adipose tissue. I. Beta 1-and beta 2-adrenergic receptors and cyclic adenosine 3′,5′-monophosphate generation. Endocrinology. 1995;136:3267–76. doi: 10.1210/endo.136.8.7628360. [DOI] [PubMed] [Google Scholar]
- 13.Wahrenberg H, Wennlund A, Arner P. Adrenergic regulation of lipolysis in fat cells from hyperthyroid and hypothyroid patients. J Clin Endocrinol Metab. 1994;78:898–903. doi: 10.1210/jcem.78.4.8157718. [DOI] [PubMed] [Google Scholar]
- 14.Jansen MS, Cook GA, Song S, Park EA. Thyroid hormone regulates carnitine palmitoyltransferase Ialpha gene expression through elements in the promoter and first intron. J Biol Chem. 2000;275:34989–97. doi: 10.1074/jbc.M001752200. [DOI] [PubMed] [Google Scholar]
- 15.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2006;29(suppl):S43–8. [PubMed] [Google Scholar]
- 16.Jequier E, Acheson K, Schutz Y. Assessment of energy expenditure and fuel utilization in man. Annu Rev Nutr. 1987;7:187–208. doi: 10.1146/annurev.nu.07.070187.001155. [DOI] [PubMed] [Google Scholar]
- 17.al Adsani H, Hoffer LJ, Silva JE. Resting energy expenditure is sensitive to small dose changes in patients on chronic thyroid hormone replacement. J Clin Endocrinol Metab. 1997;82:1118–25. doi: 10.1210/jcem.82.4.3873. [DOI] [PubMed] [Google Scholar]
- 18.Astrup A, Buemann B, Christensen NJ, et al. The contribution of body composition, substrates, and hormones to the variability in energy expenditure and substrate utilization in premenopausal women. J Clin Endocrinol Metab. 1992;74:279–86. doi: 10.1210/jcem.74.2.1530952. [DOI] [PubMed] [Google Scholar]
- 19.Svendsen OL, Hassager C, Christiansen C. Impact of regional and total body composition and hormones on resting energy expenditure in overweight postmenopausal women. Metabolism. 1993;42:1588–91. doi: 10.1016/0026-0495(93)90155-h. [DOI] [PubMed] [Google Scholar]
- 20.Abbott WG, Howard BV, Christin L, et al. Short-term energy balance: relationship with protein, carbohydrate, and fat balances. Am J Physiol. 1988;255:E332–7. doi: 10.1152/ajpendo.1988.255.3.E332. [DOI] [PubMed] [Google Scholar]
- 21.Michalaki MA, Vagenakis AG, Leonardou AS, et al. Thyroid function in humans with morbid obesity. Thyroid. 2006;16:73–8. doi: 10.1089/thy.2006.16.73. [DOI] [PubMed] [Google Scholar]
- 22.Chomard P, Vernhes G, Autissier N, Debry G. Serum concentrations of total T4, T3, reverse T3 and free T4, T3 in moderately obese patients. Hum Nutr Clin Nutr. 1985;39:371–8. [PubMed] [Google Scholar]
- 23.Eden S, Jagenburg R, Lindstedt G, Lundberg PA, Mellstrom D. Interrelationships among body mass, thyrotropin, thyroid hormones, and thyroid-hormone binding proteins in healthy 70-year-old men. Clin Chem. 1984;30:681–6. [PubMed] [Google Scholar]
- 24.Knudsen N, Laurberg P, Rasmussen LB, et al. Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. J Clin Endocrinol Metab. 2005;90:4019–24. doi: 10.1210/jc.2004-2225. [DOI] [PubMed] [Google Scholar]
- 25.Sari R, Balci MK, Altunbas H, Karayalcin U. The effect of body weight and weight loss on thyroid volume and function in obese women. Clin Endocrinol (Oxf) 2003;59:258–62. doi: 10.1046/j.1365-2265.2003.01836.x. [DOI] [PubMed] [Google Scholar]
- 26.Iacobellis G, Ribaudo MC, Zappaterreno A, Iannucci CV, Leonetti F. Relationship of thyroid function with body mass index, leptin, insulin sensitivity and adiponectin in euthyroid obese women. Clin Endocrinol (Oxf) 2005;62:487–91. doi: 10.1111/j.1365-2265.2005.02247.x. [DOI] [PubMed] [Google Scholar]
- 27.Kok P, Roelfsema F, Frolich M, Meinders AE, Pijl H. Spontaneous diurnal thyrotropin secretion is enhanced in proportion to circulating leptin in obese premenopausal women. J Clin Endocrinol Metab. 2005;90:6185–91. doi: 10.1210/jc.2005-0003. [DOI] [PubMed] [Google Scholar]
- 28.Stokholm KH, Lindgreen P. Serum free triiodothyronine in obesity. Int J Obes. 1982;6:573–8. [PubMed] [Google Scholar]
- 29.Nyrnes A, Jorde R, Sundsfjord J. Serum TSH is positively associated with BMI. Int J Obes (Lond) 2006;30:100–5. doi: 10.1038/sj.ijo.0803112. [DOI] [PubMed] [Google Scholar]
- 30.Guo F, Bakal K, Minokoshi Y, Hollenberg AN. Leptin signaling targets the thyrotropin-releasing hormone gene promoter in vivo. Endocrinology. 2004;145:2221–7. doi: 10.1210/en.2003-1312. [DOI] [PubMed] [Google Scholar]
- 31.Kim MS, Small CJ, Stanley SA, et al. The central melanocortin system affects the hypothalamo-pituitary thyroid axis and may mediate the effect of leptin. J Clin Invest. 2000;105:1005–11. doi: 10.1172/JCI8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mantzoros CS, Ozata M, Negrao AB, et al. Synchronicity of frequently sampled thyrotropin (TSH) and leptin concentrations in healthy adults and leptin-deficient subjects: evidence for possible partial TSH regulation by leptin in humans. J Clin Endocrinol Metab. 2001;86:3284–91. doi: 10.1210/jcem.86.7.7644. [DOI] [PubMed] [Google Scholar]
- 33.Rosenbaum M, Goldsmith R, Bloomfield D, et al. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest. 2005;115:3579–86. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farooqi IS, Matarese G, Lord GM, et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110:1093–103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. 2002;23:38–89. doi: 10.1210/edrv.23.1.0455. [DOI] [PubMed] [Google Scholar]
- 36.Christoffolete MA, Ribeiro R, Singru P, et al. Atypical expression of type 2 iodothyronine deiodinase in thyrotrophs explains the thyroxine-mediated pituitary thyrotropin feedback mechanism. Endocrinology. 2006;147:1735–43. doi: 10.1210/en.2005-1300. [DOI] [PubMed] [Google Scholar]
- 37.Silva JE, Larsen PR. Contributions of plasma triiodothyronine and local thyroxine monodeiodination to triiodothyronine to nuclear triiodothyronine receptor saturation in pituitary, liver, and kidney of hypothyroid rats. Further evidence relating saturation of pituitary nuclear triiodothyronine receptors and the acute inhibition of thyroid-stimulating hormone release. J Clin Invest. 1978;61:1247–59. doi: 10.1172/JCI109041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maia AL, Kim BW, Huang SA, Harney JW, Larsen PR. Type 2 iodothyronine deiodinase is the major source of plasma T3 in euthyroid humans. J Clin Invest. 2005;115:2524–33. doi: 10.1172/JCI25083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Canani LH, Capp C, Dora JM, et al. The type 2 deiodinase A/G (Thr92Ala) polymorphism is associated with decreased enzyme velocity and increased insulin resistance in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2005;90:3472–8. doi: 10.1210/jc.2004-1977. [DOI] [PubMed] [Google Scholar]
- 40.Peeters RP, van den Beld AW, Attalki H, et al. A new polymorphism in the type II deiodinase gene is associated with circulating thyroid hormone parameters. Am J Physiol Endocrinol Metab. 2005;289:E75–81. doi: 10.1152/ajpendo.00571.2004. [DOI] [PubMed] [Google Scholar]
- 41.Beckett GJ, Beddows SE, Morrice PC, Nicol F, Arthur JR. Inhibition of hepatic deiodination of thyroxine is caused by selenium deficiency in rats. Biochem J. 1987;248:443–7. doi: 10.1042/bj2480443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dumitrescu AM, Liao XH, Abdullah MS, et al. Mutations in SECISBP2 result in abnormal thyroid hormone metabolism. Nat Genet. 2005;37:1247–52. doi: 10.1038/ng1654. [DOI] [PubMed] [Google Scholar]
- 43.Brzezinska-Slebodzinska E, Pietras B. The protective role of some antioxidants and scavengers on the free radicals-induced inhibition of the liver iodothyronine 5′-monodeiodinase activity and thiols content. J Physiol Pharmacol. 1997;48:451–9. [PubMed] [Google Scholar]
- 44.Zeold A, Doleschall M, Haffner MC, et al. Characterization of the NF-κB responsiveness of the human dio2 gene. Endocrinology. 2006;147:4419–29. doi: 10.1210/en.2005-1608. [DOI] [PubMed] [Google Scholar]
- 45.Danforth E, Jr, Horton ES, O’Connell M, et al. Dietary-induced alterations in thyroid hormone metabolism during overnutrition. J Clin Invest. 1979;64:1336–47. doi: 10.1172/JCI109590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gavin LA, Moller M, McMahon F, Gulli R, Cavalieri RR. Carbohydrate reactivation of thyroxine 5′-deiodinase (type II) in cultured mouse neuroblastoma cells is dependent upon new protein synthesis. Endocrinology. 1989;124:635–41. doi: 10.1210/endo-124-2-635. [DOI] [PubMed] [Google Scholar]
- 47.Rosenbaum M, Hirsch J, Murphy E, Leibel RL. Effects of changes in body weight on carbohydrate metabolism, catecholamine excretion, and thyroid function. Am J Clin Nutr. 2000;71:1421–32. doi: 10.1093/ajcn/71.6.1421. [DOI] [PubMed] [Google Scholar]
- 48.Watanabe M, Houten SM, Mataki C, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–9. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 49.Katzeff HL, Yang MU, Presta E, Leibel RL, Hirsch J, Van Itallie TB. Calorie restriction and iopanoic acid effects on thyroid hormone metabolism. Am J Clin Nutr. 1990;52:263–6. doi: 10.1093/ajcn/52.2.263. [DOI] [PubMed] [Google Scholar]
- 50.Tataranni PA, Young JB, Bogardus C, Ravussin E. A low sympathoadrenal activity is associated with body weight gain and development of central adiposity in Pima Indian men. Obes Res. 1997;5:341–7. doi: 10.1002/j.1550-8528.1997.tb00562.x. [DOI] [PubMed] [Google Scholar]
- 51.Acheson KJ, Ravussin E, Schoeller DA, et al. Two-week stimulation or blockade of the sympathetic nervous system in man: influence on body weight, body composition, and twenty four-hour energy expenditure. Metabolism. 1988;37:91–8. doi: 10.1016/0026-0495(88)90035-2. [DOI] [PubMed] [Google Scholar]
- 52.Curcio-Morelli C, Zavacki AM, Christofollete M, et al. Deubiquitination of type 2 iodothyronine deiodinase by von Hippel-Lindau protein-interacting deubiquitinating enzymes regulates thyroid hormone activation. J Clin Invest. 2003;112:189–96. doi: 10.1172/JCI18348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Silva JE, Larsen PR. Adrenergic activation of triiodothyronine production in brown adipose tissue. Nature. 1983;305:712–3. doi: 10.1038/305712a0. [DOI] [PubMed] [Google Scholar]
- 54.Bray GA. Reciprocal relation of food intake and sympathetic activity: experimental observations and clinical implications. Int J Obes Relat Metab Disord. 2000;24(suppl):S8–17. doi: 10.1038/sj.ijo.0801269. [DOI] [PubMed] [Google Scholar]
- 55.Surks MI, Goswami G, Daniels GH. The thyrotropin reference range should remain unchanged. J Clin Endocrinol Metab. 2005;90:5489–96. doi: 10.1210/jc.2005-0170. [DOI] [PubMed] [Google Scholar]
- 56.Himms-Hagen J. Exercise in a pill: feasibility of energy expenditure targets. Curr Drug Targets CNS Neurol Disord. 2004;3:389–409. doi: 10.2174/1568007043337076. [DOI] [PubMed] [Google Scholar]


