Abstract
Background/aim
Retinal flecks are commonly observed in both Stargardt disease and fundus flavimaculatus (FFM). The aim was to determine the precise localisation of these flecks within the retinal layers using Stratus optical coherence tomography (OCT).
Methods
A prospective observational case series. A complete ophthalmological examination, including autofluorescence, fluorescein angiography (FA), and Stratus OCT (Carl Zeiss) was performed in 49 eyes of 26 consecutive patients with FFM. Six to 12 Stratus OCT linear scans focused on the retinal flecks were performed in each eye.
Results
The age at presentation ranged from 23 years to 71 years and visual acuity ranged from 20/20 to 20/400. Hyper‐reflective deposits classified into two types were observed on Stratus OCT: type 1 lesions (94% of eyes) presented as dome‐shaped deposits located in the inner part of the retinal pigment epithelium (RPE) layer and type 2 lesions (86% of eyes) presented as small linear deposits located at the level of the outer nuclear layer and clearly separated from the RPE layer.
Conclusions
Stratus OCT is a non‐invasive instrument that provides new information on the location of flecks in FFM. The location of type 2 lesions is quite unusual among macular dystrophies; OCT may therefore be useful in the diagnosis of retinal flecks in some cases of FFM.
Keywords: fundus flavimaculatus, optical coherence tomography, retinal dystrophy, Stargardt disease
Stargardt disease (STGD) is an autosomal recessive macular dystrophy of childhood, characterised by juvenile onset, rapid progression, and a poor visual outcome.1,2,3 On the other hand, fundus flavimaculatus (FFM), a Stargardt‐like phenotype described by Franceschetti, is characterised by late onset and slow progression.4 The disease is usually termed STGD when visual acuity loss begins in the first two decades, while the term FFM is favoured when the disease begins at the end of the second decade or in the third decade. Genetic studies demonstrated a continuum between STGD and FFM, both linked with the ABCA4 gene.5,6,7 Yellowish‐white deposits called retinal “flecks” are usual features of both STGD and FFM.8,9 These flecks are small or large, extremely polymorphous, rounded, fusiform, spear‐like, pisciforms, giants, butterfly or X shaped, and can be juxtamacular or diffusely distributed in the fundus.3,10 At the beginning, the deposits appear yellowish‐white and well defined. Later, the retinal flecks become grey, fuzzy, and ill defined and are hardly visible on fundus examination, although clearly revealed by autofluorescent frames.11,12,13
The exact location of the retinal flecks within the retina is still controversial.10 Klein and Krill14 established the presence of mucopolysaccharide deposits (hyaluronic acid) in retinal cells. On the other hand, Eagle et al15 found a massive accumulation of lipofuscine (that is composed of A2‐E) in the retinal pigment epithelium (RPE) cells.
Optical coherence tomography (OCT) is a non‐invasive technique based on low interferometry that provides optical cross sectional images of the retina and morphological information similar to that obtained from histological sections. Stratus OCT is a recently introduced instrument that provides morphological information of the retina with a better resolution compared with first generation OCT instruments. Our aim was to determine the precise localisation of retinal flecks within the retina, in FFM patients, using Stratus OCT.
Patients and methods
Twenty six consecutive patients who presented to our department with FFM macular dystrophy were prospectively included in this study, in compliance with French regulations and after approval from our local ethics committee. Criteria for inclusion were age over 18 years, presence of retinal flecks on fundus examination, evidence of autofluorescence of the retinal flecks, and diagnosis of dark choroid on fluorescein angiography (FA). Eyes presenting with any associated macular diseases (myopia more than −8 D, angioid streaks, confluent drusen, epiretinal membrane), or complication such as choroidal neovascularisation were excluded from this study. All patients underwent a complete ophthalmological examination, including assessment of best corrected visual acuity (BCVA), fundus biomicroscopy, colour photography of the fundus (Canon 60 fundus camera, Tokyo, Japan; Topcon TRC‐50 retinal camera, Tokyo, Japan; Zeiss FF 450 plus, Carl Zeiss AG, Germany), autofluorescence frames (confocal on Heidelberg Retina Angiograph Heidelberg Engineering, Heidelberg, Germany or non confocal on Canon 60 fundus camera, Tokyo, Japan; Topcon TRC‐50 retinal camera, Tokyo, Japan; Zeiss FF 450 plus, Carl Zeiss AG, Germany), red free and frames FA (Canon 60 fundus camera, Tokyo, Japan; Topcon TRC‐50 retinal camera, Tokyo, Japan; Zeiss FF 450 plus, Carl Zeiss AG, Germany). Optical coherence tomography examination was performed with the ultimate commercial OCT unit (Stratus OCT 3000; OCT3, Carl Zeiss Meditec, Inc, Dublin, CA, USA).
For each eye, OCT examination included a minimum of six vertical or horizontal scans of 5 mm. As it was our goal to find retinal lesions, we performed up to 12 OCT scans when no lesions were observed after six scans. The OCT scans were positioned so that the cross sectional cut would go through the flecks based on colour fundus photography and fundus autofluorescence. For each scan, the shape and reflectivity of the material, its location, the reflectivity and appearance of the RPE and any retinal changes were noted.
Results
A total of 49 eyes (26 patients) were included in this study: both eyes of 23 patients and one eye of three patients. One patient (case 1, right eye, RE) presented with a unilateral epiretinal membrane and thus only the left eye was included. Two patients (case 17, RE and case 21, left eye, LE) presented with choroidal neovascularisation and, therefore, only the fellow eye was included (table 1).
Table 1 Type 1 and type 2 hyper‐reflective deposits observed in FFM patients using Stratus OCT.
| Case | Sex | Age | Eye | Visual acuity | Type 1 deposits/ total scans | Type 2 deposits/ total scans |
|---|---|---|---|---|---|---|
| 1 | M | 58 | LE | 20/40 | 4/12 | 3/12 |
| 2 | F | 23 | RE | 20/125 | 7/12 | 6/12 |
| LE | 20/160 | 3/12 | 0/12 | |||
| 3 | M | 33 | RE | 20/50 | 3/6 | 1/6 |
| LE | 20/20 | 1/6 | 2/6 | |||
| 4 | M | 41 | RE | 20/32 | 3/12 | 0/12 |
| LE | 20/50 | 1/12 | 2/12 | |||
| 5 | F | 23 | RE | 20/125 | 6/12 | 3/12 |
| LE | 20/160 | 5/12 | 2/12 | |||
| 6 | M | 59 | RE | 20/25 | 2/6 | 0/6 |
| LE | 20/40 | 3/6 | 4/6 | |||
| 7 | M | 26 | RE | 20/160 | 5/12 | 3/12 |
| LE | 20/160 | 5/12 | 3/12 | |||
| 8 | M | 26 | RE | 20/320 | 2/6 | 6/6 |
| LE | 20/250 | 2/6 | 4/6 | |||
| 9 | F | 41 | RE | 20/80 | 5/12 | 4/12 |
| LE | 20/63 | 4/12 | 4/12 | |||
| 10 | M | 47 | RE | 20/40 | 3/12 | 1/12 |
| LE | 20/50 | 2/12 | 1/12 | |||
| 11 | M | 66 | RE | 20/25 | 4/6 | 6/6 |
| LE | 20/32 | 1/6 | 3/6 | |||
| 12 | F | 44 | RE | 20/40 | 4/6 | 2/6 |
| LE | 20/20 | 1/6 | 3/6 | |||
| 13 | F | 33 | RE | 20/80 | 5/10 | 1/10 |
| LE | 20/63 | 5/12 | 5/12 | |||
| 14 | M | 38 | RE | 20/63 | 3/12 | 0/12 |
| LE | 20/200 | 3/6 | 3/6 | |||
| 15 | M | 51 | RE | 20/40 | 6/6 | 6/6 |
| LE | 20/32 | 6/6 | 6/6 | |||
| 16 | M | 71 | RE | 20/40 | 5/7 | 6/7 |
| LE | 20/160 | 10/12 | 9/12 | |||
| 17 | M | 58 | LE | 20/40 | 10/12 | 9/12 |
| 18 | F | 24 | RE | 20/200 | 7/12 | 10/12 |
| LE | 20/200 | 5/12 | 7/12 | |||
| 19 | F | 55 | RE | 20/20 | 2/12 | 4/12 |
| LE | 20/400 | 0/12 | 6/12 | |||
| 20 | F | 41 | RE | 20/32 | 3/6 | 1/6 |
| LE | 20/32 | 2/6 | 1/6 | |||
| 21 | F | 70 | RE | 20/25 | 4/6 | 3/6 |
| 22 | M | 26 | RE | 20/50 | 2/10 | 0/10 |
| LE | 20/63 | 5/10 | 2/10 | |||
| 23 | M | 45 | RE | 20/25 | 8/10 | 7/10 |
| LE | 20/25 | 7/10 | 6/10 | |||
| 24 | M | 32 | RE | 20/63 | 3/6 | 2/6 |
| LE | 20/25 | 0/12 | 1/12 | |||
| 25 | M | 58 | RE | 20/400 | 0/12 | 1/12 |
| LE | 20/63 | 2/12 | 0/12 | |||
| 26 | M | 29 | RE | 20/100 | 5/6 | 2/6 |
| LE | 20/63 | 5/6 | 0/6 |
M: male; F: female; RE: right eye; LE: left eye.
There were nine women and 17 men with a mean age at presentation of 43 years (range 23–71 years). BCVA ranged from 20/20 to 20/400 and was better than 20/40 in 14 eyes, between 20/40 and 20/80 in 20 eyes, and less than 20/80 in 15 eyes (table 1).
On colour fundus photography, the retinal flecks presented heterogeneous patterns, which were perifoveolar or widely distributed in the fundus. These retinal flecks were more prominently visible in the red free frames in comparison with the colour photographs. Autofluorescent frames clearly delineated the retinal flecks. On FA, the retinal flecks appeared as ill defined areas of hypofluorescence, surrounded by haloes of hyperfluorescence corresponding to changes in the RPE. In accordance with the strict criteria for inclusion, dark choroid was present in all the study eyes.
Stratus OCT allowed visualisation of hyper‐reflective dots that could be interpreted as the presence of material that makes up the retinal flecks. When comparing Stratus OCT scans with colour photographs of the fundus and autofluorescent frames, the location of the hyper‐reflective deposits matched the location of the retinal flecks in all eyes. Although all the OCT scan sections appeared to include the retinal flecks, the hyper‐reflective lesions were not detectable in all the OCT sections. However, performing 6–12 scans per eye allowed the identification of the hyper‐reflective deposits in all the study eyes.
These hyper‐reflective deposits were located more or less deeply within the retinal layers. The hyper‐reflective deposits were classified into two groups, according to their apparent location within the retina on Stratus OCT. Type 1 deposits were dome‐shaped and aspect located at the level of or just above the RPE in continuum with the inner part of the RPE layer (fig 1). In most cases, the reflectivity of the RPE and the material were very close and, hence, could not be differentiated by OCT. Type 1 deposits were observed in at least one OCT scan in 46 out of 49 (94%) eyes and in two or more OCT scans in 42 out of 49 (86%) eyes (table 1). Type 2 deposits presented as small, linear, hyper‐reflective lesions located at the level of the inner segments of photoreceptors or outer nuclear layer (ONL) and clearly separated from the RPE layer (figs 2 and 3). The OCT reflectivity of type 2 deposits was similar to the reflectivity of type 1 deposits, in contrast with the hyporeflectivity of the ONL. Type 2 lesions were observed in at least one OCT scan in 42 out of 49 (86%) eyes and in two or more OCT scans in 34 out of 49 (69%) eyes.
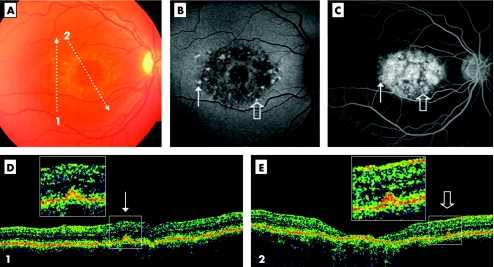
Figure 1 Stratus optical coherent tomography (OCT) demonstrates type 1 deposits facing retinal flecks (case 5, RE). Colour photograph shows macular atrophy and retina flecks (A). OCT scans are illustrated by the arrows 1 and 2. The autofluorescent frame (confocal) clearly delineates the retinal flecks (B). Thin and open arrows point out two of the flecks, respectively crossed by scans 1 and 2. Fluorescein angiography shows the dark choroid appearance (C). The hypofluorescent flecks (arrows) are hardly discernable in the hyperfluorescent background because of retinal pigment epithelium (RPE) changes. The OCT scan 1 (D) shows a small hyper‐reflective lesion located at the inner part of the RPE layer (thin arrow), called type 1 deposit. The OCT scan 2 (E) shows a similar feature (open arrow) and macular atrophy. Squares illustrate enlarged view of these little dots that appear to correspond to the accumulation of autofluorescent material marked by the arrows.
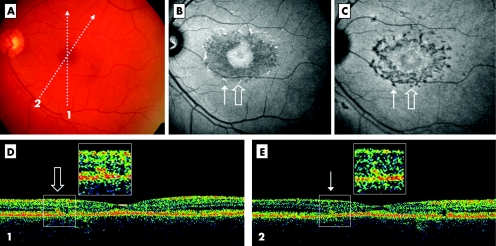
Figure 2 Stratus OCT demonstrates type 2 deposits facing retinal flecks (case 12, LE). Colour photograph shows macular atrophy and retina flecks (A). OCT scans are illustrated by the arrows 1 and 2. The autofluorescent frame (non‐confocal) clearly delineates the retinal flecks and shows autofluorescence of the macular area (B). Arrows point out two of the flecks, crossed by scans 1 and 2. On indocyanine green angiography (ICG), late phase (30 min), the hypofluorescent lesions appear more numerous than the autofluorescent flecks (C). The OCT scan 1 (D) shows a small hyper‐reflective linear lesion located at the level of the outer nuclear layer and clearly distinguished from the RPE layer (open arrow), called type 2 deposit. The OCT scan 2 (E) shows a similar feature (thin arrow). These small lesions appear to correspond to the accumulation of auto‐fluorescent material and are interpreted as retinal flecks marked by the arrows. It is notable that only the autofluorescent flecks could be identified by OCT.
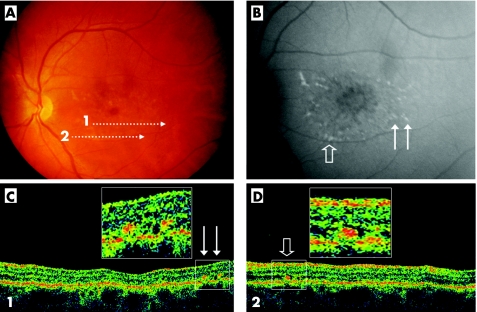
Figure 3 Example of type 2 deposits evidenced by Stratus OCT (case 9, LE). Colour photograph shows macular atrophy and retinal flecks. OCT scans are illustrated by the arrows 1 and 2. The autofluorescent frame (non confocal) demarcates the retinal flecks and shows autofluorescence of the macular area (B). Arrows point out three of the flecks, crossed by scans 1 (thin arrows) and 2 (open arrow). The OCT scan 1 (C) shows two small hyper‐reflective dots localised at the level of the outer nuclear layer (thin arrows), called type 2 deposits. The hyper‐reflective deposits observed at the level of the ONL by Stratus OCT appears to correspond to retinal flecks (open arrow; D).
All the study eyes presented at last one of the two described patterns of hyper‐reflective deposits on OCT. When both eyes were combined, all the study patients presented both types of lesions on Stratus OCT (fig 4).
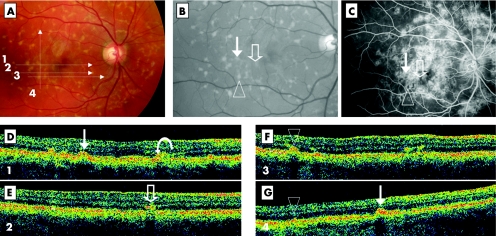
Figure 4 Both type 1 and type 2 FFM deposits visualised by Stratus OCT within the same eye (case 12, LE). Colour photograph shows numerous retinal flecks (A). The 5 mm OCT scans are illustrated by the four numbered arrows. In the red free frame (enlarged view), the flecks appear slightly more contrasted (B). No profound macular atrophy is visible. Thin arrow, open arrow, and arrowhead point out three of the flecks crossed by the OCT scans. Fluorescein angiography shows ill defined hyperfluorescence around the macular area and a dark choroid appearance on the temporal side (C). Arrows are marked on the corresponding locations. On OCT scan 1 (D), a dome‐shaped elevation of the RPE level (type 1, thin arrow) is observed. On the same scan, two other lesions are visible: a deposit within the photoreceptor layer (type 2, on the left side of the arrow) and a lesion, intermediate between the type 1 and type 2 patterns (curved arrow). On OCT scan 2 (E), a typical type 2 deposit is observed, clearly distinct from the RPE layer (open arrow). This hyper‐reflective deposit does not clearly correlate with the well defined flecks on colour photography but could correspond to a hypofluorescent lesion on fluorescein angiography (open arrow). On OCT scan 3 (F), a type 1 deposit is observed (triangle) as well as two small type 2 deposits on the right side, suggesting heterogeneous aspect of the same lesion type. On OCT scan 4 (G) the type 1 deposits corresponding to the arrowhead and the thin arrow are shown again.
Discussion
In this series of FFM patients, Stratus OCT revealed small hyper‐reflective deposits corresponding with the retinal flecks located either at the level of the RPE layer or at the level of the ONL.
Retinal flecks observed in STGD/FFM patients are typically heterogeneous, in their surface, their pattern, and their distribution in the fundus. On fundus examination and colour photography, these flecks are commonly poorly contrasted and appear as yellowish‐white lesions on an orange background. The retinal flecks are clearer on red free frames, although they are best and clearly delineated on autofluorescent frames (fig 4B).16
Despite some histological reports, the exact location of the retinal flecks within the retinal layers remains controversial. Recently, Ergun et al17 analysed photoreceptor morphology in 14 patients affected with Stargardt's disease and fundus flavimaculatus using ultra high resolution optical coherence tomography (UHR‐OCT). In this series, UHR‐OCT demonstrated excellent visualisation of intraretinal morphology and enabled quantification of the photoreceptor layer. Lower visual acuity correlated with a greater transverse photoreceptor loss. However, they did not demonstrate the location of the retinal flecks. Here our purpose was to determine the precise location of the retinal flecks within the retina in FFM patients.
On Stratus OCT, the retinal flecks presented as small hyper‐reflective lesions located either at the inner part of the RPE layer (type 1) or at the level of the ONL (type 2). None of the fundus features, the features on autofluorescent frames, or angiographic features were characteristic of a particular type of retinal flecks. In other words, neither of the two types of hyper‐reflective lesions was correlated with a specific phenotype of the flecks.
Type 1 lesions were observed in 94% (46 out of 49) of the study eyes and all the 26 patients presented this type of lesions in at least one eye. They appeared as dome‐shaped hyper‐reflective deposits in the inner part of the RPE layer (figs 1 and 4). This finding may be compared with that observed in adult onset foveomacular vitelliform dystrophy (AFVD).18 However, the hyper‐reflective lesions are smaller and dome‐shaped in FFM contrasting with the stretch aspect observed in AFVD.
Type 2 lesions were observed in 86% (42 out of 49) of the study eyes and all 26 patients presented this type of lesions in at least one eye. It is conceivable that among eyes in which type 2 lesions was not identified, performing more scans or 3 mm scans may have revealed the “absent” lesion. Type 2 lesions appeared as small, linear, hyper‐reflective deposits at the level of the ONL and well differentiated visibly from the RPE layer (figs 2–4). This location is unusual in the field of inherited macular dystrophies distinct from lesions observed in AFVD,18 Best macular dystrophy,19 or malattia leventinese,20 localised at the level of RPE We believe that this unusual location could contribute to the positive diagnosis in some cases of retinal flecks in STGD/FFM.
Several lines of evidence suggest that the two types of retinal flecks merely reflect different stages of the same disorder. Firstly, both lesions were observed simultaneously in 80% (39 out of 49) of the study eyes. Secondly, all patients had the two lesion types, either in the right eye or the left eye. Finally, in some scan sections, intermediate aspect, sharing common features between types 1 and 2 were observed (fig 4D).
However, the chronological evolution, from type 1 to type 2 or from type 2 to type 1, could be debated. On the one hand, it could be speculated that type 2 deposits progressively increase from the photoreceptor layer to the RPE. This hypothesis would be consistent with the fact that the initial impairment, the ABCA4 protein, is primarily located in the photoreceptors. On the other hand, and most likely, we hypothesise that the hyper‐reflective deposits at the level of the ONL (type 2) could be the residual cover of the dome‐shaped lesions (type 1). This latter hypothesis is consistent with the natural history of the flecks in the fundus; these flecks progressively degrade, from a well defined lesion to residual material.
Alternatively, these small hyper‐reflective lesions could be regarded as one lesion type which appears to be different because of the resolution of Stratus OCT. It could be considered that the main difference between type 1 and type 2 deposits is the visualisation or not of a continuum with the RPE layer.
In conclusion, Stratus OCT is a useful non‐invasive instrument that provides new information on the location of the retinal flecks in STGD/FFM. The yellowish‐white material is located either at the level of the RPE (type 1) or, unusually, at the level of the outer nuclear layer (type 2). We believe that OCT may provide additional diagnostic information and could help in the diagnosis of retinal flecks in some cases of STGD/FFM owing to the unusual location of the type 2 lesions.
Abbreviations
AFVD - adult onset foveomacular vitelliform dystrophy
BCVA - best corrected visual acuity
FFM - fundus flavimaculatus
OCT - optical coherence tomography
ONL - outer nuclear layer
RPE - retinal pigment epithelium
STGD - Stargardt disease
UHR‐OCT - ultra high resolution optical coherence tomography
Footnotes
The authors have no proprietary interest in the materials used in this study.
References
- 1.Stargardt K. Ueber familiare progressive degeneration in der makulagegend des Auges. Albrecht V Graefes Arch Ophthalmol 190971534–550. [Google Scholar]
- 2.Cibis G W, Morey M, Karris D J. Dominantly inherited macular dystrophy with flecks (Stargardt). Arch Ophthalmol 1980981785. [DOI] [PubMed] [Google Scholar]
- 3.Merlin S, Landau J. Abnormal findings in relatives of patients with juvenile hereditary macular degeneration (Stargardt's disease). Ophthalmologica 19701611. [DOI] [PubMed] [Google Scholar]
- 4.Franceschetti A, Francois J. Fundus flavimaculatus. Arch Ophthalmol 196525505. [PubMed] [Google Scholar]
- 5.Allikmets R, Singh N, Sun H.et al A photoreceptor cell‐specific ATP‐binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet 199715236–246. [DOI] [PubMed] [Google Scholar]
- 6.Cremers F P, van de Pol D J, van Driel M.et al Autosomal recessive retinitis pigmentosa and cone‐rod dystrophy caused by splice site mutations in the Stargardt's disease gene ABCR. Hum Mol Genet 19987355–362. [DOI] [PubMed] [Google Scholar]
- 7.Rozet J M, Gerber S, Souied E.et al Spectrum of ABCR gene mutations in autosomal recessive macular dystrophies. Eur J Hum Genet 19986291–295. [DOI] [PubMed] [Google Scholar]
- 8.Klien B A, Krill A E. Fundus flavimaculatus: clinical, functional and histopathologic observations. Am J Ophthalmol 1967643. [PubMed] [Google Scholar]
- 9.Aaberg T M. Stargardt's disease and fundus flavimaculatus: evaluation of morphologic progression and intrafamilial co‐existence. Trans Am Ophthalmol Soc 198084453–487. [PMC free article] [PubMed] [Google Scholar]
- 10.Lois N, Holder G E, Bunce C.et al Phenotypic subtypes of Stargardt macular dystrophy‐fundus flavimaculatus. Arch Ophthalmol 2001119359–369. [DOI] [PubMed] [Google Scholar]
- 11.Holz F G. Autofluorescence imaging of the macula. Ophthalmologe 20019810–18. [DOI] [PubMed] [Google Scholar]
- 12.Von Ruckmann A, Fitzke F W, Bird A C. In vivo autofluorescence in macular dystrophies. Arch Ophthalmol 1997115609–615. [DOI] [PubMed] [Google Scholar]
- 13.Souied E, Kaplan J, Coscas G.et al Macular dystrophies. J Fr Ophtalmol 200326743–762. [PubMed] [Google Scholar]
- 14.Klein B A, Krill A E. Fundus flavimaculatus. Clinical, functional and histopathological observations. Am J Ophthalmol 1967643–23. [PubMed] [Google Scholar]
- 15.Eagle R C, Lucier A C, Bernadrdino V B.et al Retinal pigment epithelial abnormalities in fundus flavimaculatus. Ophthalmology 1980871189–1200. [DOI] [PubMed] [Google Scholar]
- 16.Lois N, Halfyard A S, Bird A C.et al Fundus autofluorescence in Stargardt macular dystrophy‐fundus flavimaculatus. Am J Ophthalmol 200413855–63. [DOI] [PubMed] [Google Scholar]
- 17.Ergun E, Hermann B, Wirtitsch M.et al Assessment of central visual function in Stargardt's disease/fundus flavimaculatus with ultrahigh‐resolution optical coherence tomography. Invest Ophthalmol Vis Sci 200546310–316. [DOI] [PubMed] [Google Scholar]
- 18.Benhamou N, Souied E, Zolf R.et al Adult‐onset foveomacular vitelliform dystrophy: a study by optical coherence tomography. Am J Ophthalmol 2003135362–367. [DOI] [PubMed] [Google Scholar]
- 19.Men G, Batioglu F, Ozkan S S.et al Best's vitelliform macular dystrophy with pseudohypopion: an optical coherence tomography study. Am J Ophthalmol 2004137963–965. [DOI] [PubMed] [Google Scholar]
- 20.Souied E H, Leveziel N, Letien V.et al Optical coherent tomography features of malattia leventinese. Am J Ophthalmol 2006141404–407. [DOI] [PubMed] [Google Scholar]