Abstract
Aims
To investigate optic nerve head topography in patients with optic neuritis compared to controls using the Heidelberg retina tomograph‐II (HRT‐II) and to determine if detected changes are related to visual function and electrophysiology.
Methods
25 patients with a previous single episode of unilateral optic neuritis and 15 controls were studied with HRT‐II, visual evoked potentials, and pattern electroretinogram. Patients also had testing of visual acuity, visual field, and colour vision.
Results
In affected eyes compared to fellow eyes, there was reduction of both the mean retinal nerve fibre layer (RNFL) thickness at the disc edge (p = 0.009) and the neuroretinal rim volume (p = 0.04). In affected eyes compared to control eyes, the three dimensional optic cup shape measure was increased (p = 0.01), indicative of an abnormal cup shape. There were no other significant differences in HRT‐II measures. Within patient interocular difference correlation was used to investigate the functional relevance of these changes and demonstrated associations between RNFL thickness change and changes in visual acuity, visual field, and colour vision. Colour vision change was also associated with change in neuroretinal rim volume.
Conclusions
HRT detects functionally relevant changes in RNFL thickness and neuroretinal rim volume between eyes affected by optic neuritis and unaffected fellow eyes.
Keywords: optic neuritis, optic nerve head, scanning laser tomography, axonal loss, multiple sclerosis
A substantial proportion of relapses in multiple sclerosis (MS) have incomplete recovery,1 which is now thought to be related to axonal loss. Optic neuritis is a useful model for studying relapses in MS as it is a typical first presentation of MS and can also occur as a relapse in the established disease. Furthermore, the pathology of the optic neuritis lesion is the same as in other forms of MS relapse. Non‐invasive optic nerve magnetic resonance imaging (MRI), combined with electrophysiological and clinical measures of anterior visual pathway function have provided insights into the pathophysiological mechanisms associated with relapse and recovery in optic neuritis.2,3,4 Measurement of the retinal nerve fibre layer (RNFL) using optical coherence tomography (OCT) detects functionally relevant axonal loss5,6 and may be useful for monitoring in future trials of neuroprotective therapies in optic neuritis and MS.
Optic disc photography can provide two dimensional data on neuroretinal rim area, cup area, and cup:disc ratio. An earlier study using optic disc photography found that pathological optic disc cupping was present in 16% of eyes affected by optic neuritis,7 whereas, in contrast, a similar study found no significant increase in optic disc cupping.8 In another study, measurements of the neuroretinal rim area were taken from optic disc photographs of patients with previous optic neuritis and 37% were classed as abnormal.9 Findings from these studies using disc photography, although informative, are not quantitative, are prone to measurement error, and produce anatomical data in only two dimensions.
Scanning laser tomography10 performed with the Heidelberg retina tomograph II (HRT‐II) can be used clinically to produce three dimensional images of the optic nerve head from which a number of anatomical measures can be obtained.11 These include measurements of the optic disc, cup, neuroretinal rim, and RNFL. Although the main current clinical application for the HRT‐II is to monitor progression of glaucomatous changes over time, it could potentially be used to study optic nerve head topography in other optic neuropathies.
In this study, the HRT‐II was used to study a cohort of patients with a single clinically symptomatic episode of optic neuritis to determine (i) whether there are any quantifiable changes in optic nerve head topography, and (ii) whether any observed changes in the optic nerve head topography are related to functional outcome.
Methods
This study had local ethics committee approval and informed consent in writing was obtained from all subjects, in accordance with the Declaration of Helsinki.
Subjects
Patients
Twenty five patients with only a single previous symptomatic attack of acute unilateral optic neuritis and no recurrence were recruited from Moorfields Eye Hospital, London, with a selection bias towards those with incomplete visual recovery. The definition of a single unilateral attack is purely clinical and the possibility that an asymptomatic recurrent attack may have occurred in the initially affected eye can not be excluded. In addition, the absence of clinical involvement in the fellow eyes does not preclude the possibility of subclinical involvement, and there was no exclusion according to examination findings of the fellow eye. The diagnosis of optic neuritis was made by an experienced neuro‐ophthalmologist (GTP) and appropriate investigations including anterior segment biomicroscopy, measurement of intraocular pressure, and blood tests for a systemic inflammatory/infective aetiology had excluded alternative diagnoses. All patients had optic disc pallor and a relative afferent pupillary defect, which was not quantified further. The minimum interval from onset of optic neuritis to the time of examination was 1 year: this meant that there was no residual disc swelling in the anterior optic neuritis cases which would have interfered with HRT‐II measurements, and also meant that visual function had almost certainly stabilised. The mean age was 40 years (range 22–57 years) with 11 males and 14 females. Both the mean and median duration since onset of optic neuritis was 3 years (range 1–9 years).
Controls
Fifteen control subjects were recruited with a mean age of 36 years (range 30–56) and consisted of six males and nine females. None of the controls were known to have any ophthalmological or neurological disorder.
Imaging
Optic nerve head imaging of both eyes was performed using the HRT‐II device (Heidelberg Engineering, Germany) and the Heidelberg Eye Explorer (version 1.7) operating system to generate mean topography images and to perform image analysis using the 320 μm reference plane. Pupils were not dilated. Scans were performed at the Glaucoma Research Unit, Moorfields Eye Hospital. Optic disc contour lines were all drawn by the same experienced observer (PGS) who was masked to the clinical status of each subject.
Visual testing
The logMAR visual acuity of patients with appropriate refraction was obtained.12 The visual field was tested using the full threshold 30–2 program on the Humphrey field analyser to produce the mean deviation (MD). One patient was unable to reliably perform automated perimetry because of high levels of fixation losses and false negative responses. Colour vision was assessed using the Farnsworth‐Munsell 100‐Hue test13 and scored as square root of the error score (√FM 100‐Hue score) because this provides a nearer normal distribution.14 Two patients with a congenital anomaly of colour vision were excluded from this test.
Electrophysiology
Monocular stimuli were used to record visual evoked potentials (VEP). Two repetitions of each VEP were recorded to ensure reproducibility, and subsequently averaged together. The stimuli comprised reversal of a checkerboard pattern in the “whole field” and in the “central field” as previously described by Brusa et al,15 where a detailed description of the VEP methodology can be found. Amplitudes and latencies from the whole and central fields were considered for analysis. Central field responses were unobtainable in one patient and one control.
The pattern electroretinogram (PERG) was recorded to binocular stimulation of the “whole field,” subtending 28° horizontally by 20° vertically. A detailed description of the methodology has been provided previously by Trip et al.5 The N95 amplitude and latency were considered for analysis.
Analysis was performed blind to the status of each subject.
Statistical analysis
Data were expressed as absolute values for affected and clinically unaffected fellow eyes of patients, and also for one randomly selected eye from each control. Statistical analyses were performed using SPSS 11.0 for Windows (SPSS, Chicago, IL, USA). The parameters appeared to follow a normal distribution, therefore t tests were employed. Differences in the HRT‐II measures between optic nerves from patients and controls were assessed using an independent samples t test. Affected eyes and clinically unaffected fellow eyes within patients were compared using a paired t test. Where differences were found their functional relevance was determined by calculating Spearman rank correlation coefficients between the HRT‐II imaging measures, visual function, and electrophysiological variables. Associations between the HRT‐II measures and functional measures are better studied when there is correction for “inter‐subject variability” as the normal background inter‐individual variation in optic nerve head topography and visual function could mask monocular correlations. We did this by calculating the within patient interocular difference (affected eye − clinically unaffected fellow eye) for each parameter as this essentially gives a measure of disease effect within a patient. Because in some patients there will be subclinical pathology in the clinically unaffected optic nerve, this approach may provide a conservative estimate of the extent of abnormality in the affected nerve. Conversely, if one or more clinically silent recurrent episodes had occurred in the affected eye, this approach may overestimate the extent of abnormality from a single clinical episode.
Results
Visual function and electrophysiology data are presented elsewhere.5
The HRT‐II imaging data are presented in table 1.
Table 1 Optic nerve head imaging measures in control eyes, unaffected patient eyes, and affected patient eyes.
| Control eyes* (n = 15) | Unaffected patient eyes (n = 25) | Affected patient eyes (n = 25) | ||
|---|---|---|---|---|
| Optic disc area (mm2) | 1.90 (0.48) | 2.02 (0.43) | 2.04 (0.36) | |
| pc = 0.42 | pc = 0.32 | |||
| pf = 0.84 | ||||
| Optic cup area (mm2) | 0.51 (0.49) | 0.51 (0.34) | 0.62 (0.44) | |
| pc = 0.97 | pc = 0.48 | |||
| pf = 0.17 | ||||
| Cup shape measure† | −0.23 (0.07) | −0.28 (0.42) | −0.17 (0.08) | |
| pc = 0.72 | pc = 0.01 | |||
| pf = 0.26 | ||||
| Neuroretinal rim area (mm2) | 1.39 (0.31) | 1.51 (0.36) | 1.42 (0.41) | |
| pc = 0.32 | pc = 0.83 | |||
| pf = 0.21 | ||||
| Neuroretinal rim volume (mm2) | 0.38 (0.22) | 0.40 (0.16) | 0.34 (0.19) | |
| pc = 0.80 | pc = 0.50 | |||
| pf = 0.04 | ||||
| Mean RNFL thickness at the disc edge (mm) | 0.24 (0.11) | 0.23 (0.08) | 0.19 (0.08) | |
| pc = 0.82 | pc = 0.08 | |||
| pf = 0.009 |
Values are mean (SD). pc values are comparisons between controls and patients. pf values are comparisons between unaffected fellow eyes and affected eyes in patients.
*One randomly selected eye from each control subject was used. †This is a measure for the overall three dimensional shape of the optic disc cup and has no units. RNFL, retinal nerve fibre layer.
In eyes affected by optic neuritis, mean RNFL thickness was reduced by 21% (p = 0.009) and neuroretinal rim volume reduced by 15% (p = 0.04) compared to clinically unaffected fellow eyes. The cup shape measure quantifies the three dimensional shape of the optic disc cup and has no units, with more positive values indicating a more abnormal cup shape. This measure was increased in affected eyes compared to control eyes (p = 0.01).
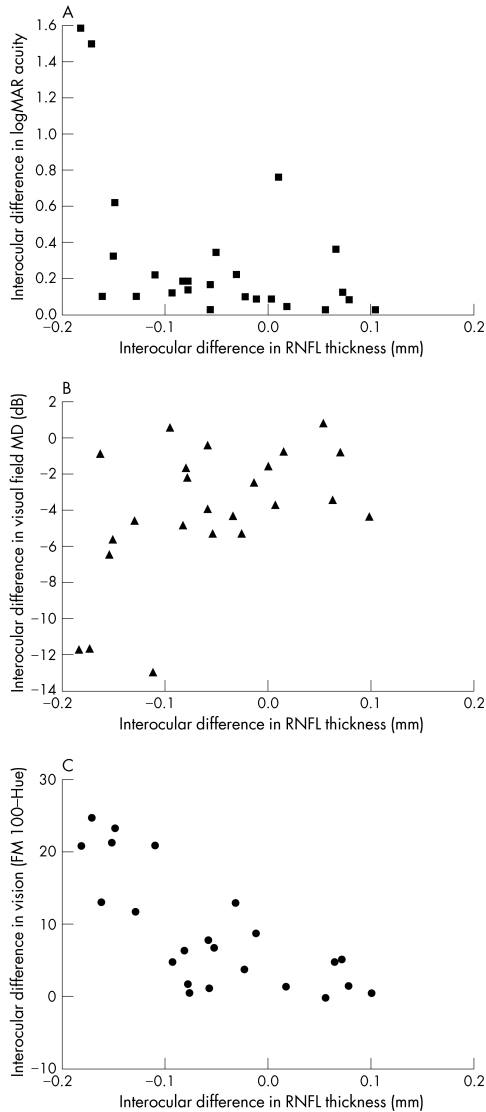
Within patient interocular correlations were explored between the above three HRT‐II measures and the measures of visual function and are presented in table 2. The correlations with mean RNFL thickness are also illustrated in figure 1. There were no correlations between HRT‐II measures and the electrophysiological parameters studied.
Table 2 Correlation of within patient interocular differences in mean RNFL thickness at the disc edge, neuroretinal rim volume and cup shape measure with interocular differences in visual function.
| Interocular difference | Mean RNFL thickness | Neuroretinal rim volume | Cup shape measure |
|---|---|---|---|
| Visual acuity (logMAR) | r = −0.51 | r = −0.32 | r = 0.26 |
| p = 0.009 | p = 0.12 | p = 0.21 | |
| Visual field (mean deviation) | r = 0.48 | r = 0.31 | r = −0.22 |
| p = 0.02 | p = 0.14 | p = 0.30 | |
| Colour vision (√FM 100‐Hue score) | r = −0.71 | r = −0.42 | r = 0.19 |
| p<0.001 | p = 0.05 | p = 0.38 |
r values are Spearman rank correlation coefficients; RNFL, retinal nerve fibre layer; MAR, minimum angle of resolution; FM, Farnsworth Munsell.
Figure 1 Interocular difference in retinal nerve fibre layer (RNFL) thickness plotted against interocular differences in (A) logMAR visual acuity, (B) visual field mean deviation (MD), and (C) colour vision (√Farnsworth Munsell Hue‐100 error score).
Discussion
Scanning laser tomography using the HRT‐II device has been shown to provide reproducible optic nerve head measurements.16 This is the first study to apply HRT imaging to optic neuritis in order to determine if any topographic changes to the optic nerve head accompany the previously described peri‐papillary RNFL thinning and macula volume loss detected with OCT.5 Caution should be applied when interpreting differences in the HRT‐II measures and correlations with visual function because of multiple testing, and results from this pilot study should be considered as “hypothesis generating.”
The HRT‐II measures RNFL thickness around the optic disc edge and therefore differs from OCT studies where a 3.4 mm diameter circular scan just outside the optic disc is used. The reduction in RNFL thickness in affected eyes compared to fellow eyes at the disc edge is of a slightly lower magnitude (21%) in the present study compared to the previous OCT study (27%) of the same cohort.5 The differing magnitude of thinning may reflect the different physical principles and higher resolution of OCT; the use of a reference plane from which the HRT‐II calculates RNFL thickness; and the different site of RNFL measurement at the disc edge. The neuroretinal rim volume—a measure of axonal tissue within the optic disc—was also reduced in affected compared to fellow eyes. This is not surprising given that the RNFL at the optic disc edge and the neuroretinal rim are adjacent and both carry optic nerve axons. The lack of significant difference in both of these measures compared to control eyes could be because affected−fellow differences represent disease effect whereas comparison with controls is confounded by inter‐individual variability, particularly with the relatively modest subject numbers in this pilot study.
No significant differences were seen in optic disc area, cup area, or neuroretinal rim area; these measures may not reflect axonal loss as directly as RNFL thickness and neuroretinal rim volume. The lack of difference in rim area to accompany the change in rim volume could be related to the surface contour. If the rim surface following optic neuritis has a flat contour, axon loss may have less impact on the rim area than on rim volume.
The cup shape measure was greater in affected eyes compared to control eyes but not compared to fellow eyes; although this measure is primarily intended to detect “cupping” in glaucoma, the change seen here could be secondary to axonal loss from optic neuritis and the lack of significant difference with fellow eyes might reflect subclinical disease in the fellow eyes, which is a recognised phenomenon in unilateral optic neuritis.17
The previously reported relation between OCT detected RNFL thinning and measures of visual function5 was also seen here with HRT detected RNFL thinning at the optic disc edge when interocular differences were used to focus on disease effect. In particular, colour vision was found to show the closest correlation with HRT detected RNFL thinning, as was the case with the OCT study. This may reflect higher sensitivity of the FM 100‐Hue in detecting and stratifying colour vision defects compared to the other measures of visual function, lower measurement variability, or greater reliance of colour vision on optic nerve axon integrity (patients with fully recovered acuity following optic neuritis often have residual deficits in colour vision). However, the relation between OCT detected RNFL thinning and VEP amplitude reduction in the same study was not reproduced in the present study. There appears to be greater inter‐individual variability in the HRT measured RNFL thickness compared to the previously reported OCT measures,5 and the former may therefore be less accurate in detecting functionally relevant axonal loss
The HRT‐II could have a role alongside OCT if it can detect within patient changes indicative of axonal loss in longitudinal studies of optic neuritis. If this is confirmed by further studies, it could be used in the future to monitor the efficacy of neuroprotective therapies in optic neuritis.
Acknowledgements
We would like to thank all the subjects who took part in this study. The NMR Research Unit is supported by the Multiple Sclerosis Society of Great Britain and Northern Ireland. PGS is supported by the Guide Dogs for the Blind Association.
Abbreviations
FM - Farnsworth Munsell
HRT‐II - Heidelberg retina tomograph‐II
MD - mean deviation
MAR - minimum angle of resolution
MRI - magnetic resonance imaging
MS - multiple sclerosis
OCT - optical coherence tomography
PERG - pattern electroretinogram
RNFL - retinal nerve fibre layer
VEP - visual evoked potentials
Footnotes
Competing interests: DFGH has received research support from Heidelberg Engineering and Carl Zeiss Meditec, and is a member of the advisory board for Carl Zeiss Meditec.
References
- 1.Lublin F D, Baier M, Cutter G. Effect of relapses on development of residual deficit in multiple sclerosis. Neurology 2003611528–1532. [DOI] [PubMed] [Google Scholar]
- 2.Hickman S J, Toosy A T, Jones S J.et al Serial magnetization transfer imaging in acute optic neuritis. Brain 2004127692–700. [DOI] [PubMed] [Google Scholar]
- 3.Hickman S J, Toosy A T, Jones S J.et al A serial MRI study following optic nerve mean area in acute optic neuritis. Brain 20041272498–2505. [DOI] [PubMed] [Google Scholar]
- 4.Hickman S J, Toosy A T, Miszkiel K A.et al Visual recovery following acute optic neuritis—a clinical, electrophysiological and magnetic resonance imaging study. J Neurol 2004251996–1005. [DOI] [PubMed] [Google Scholar]
- 5.Trip S A, Schlottmann P G, Jones S J.et al Retinal nerve fiber layer axonal loss and visual dysfunction in optic neuritis. Ann Neurol 200558383–391. [DOI] [PubMed] [Google Scholar]
- 6.Fisher J B, Jacobs D A, Markowitz C E.et al Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmology. 2006: Epub ahead of print, [DOI] [PubMed]
- 7.Trobe J D, Glaser J S, Cassady J C. Optic atrophy. Differential diagnosis by fundus observation alone. Arch Ophthalmol 1980981040–1045. [DOI] [PubMed] [Google Scholar]
- 8.Radius R L, Maumenee A E. Optic atrophy and glaucomatous cupping. Am J Ophthalmol 197885145–153. [DOI] [PubMed] [Google Scholar]
- 9.MacFadyen D J, Drance S M, Douglas G R.et al The retinal nerve fiber layer, neuroretinal rim area, and visual evoked potentials in MS. Neurology 1988381353–1358. [DOI] [PubMed] [Google Scholar]
- 10.Sharp P F, Manivannan A, Xu H.et al The scanning laser ophthalmoscope—a review of its role in bioscience and medicine. Phys Med Biol 2004491085–1096. [DOI] [PubMed] [Google Scholar]
- 11. http://www.heidelbergengineering.com/
- 12.Ferris FL I I I, Kassoff A, Bresnick G H.et al New visual acuity charts for clinical research. Am J Ophthalmol 19829491–96. [PubMed] [Google Scholar]
- 13.Farnsworth D. The Farnsworth‐Munsell 100‐hue and dichotomous tests for color vision. J Opt Soc Am 194333568–578. [Google Scholar]
- 14.Kinnear P R. Proposals for scoring and assessing the 100‐Hue test. Vis Res 197010423–433. [DOI] [PubMed] [Google Scholar]
- 15.Brusa A, Jones S J, Plant G T. Long‐term remyelination after optic neuritis: a 2‐year visual evoked potential and psychophysical serial study. Brain 2001124468–479. [DOI] [PubMed] [Google Scholar]
- 16.Strouthidis N G, White E T, Owen V M.et al Factors affecting the test‐retest variability of Heidelberg retina tomograph and Heidelberg retina tomograph II measurements. Br J Ophthalmol 2005891427–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beck R W, Kupersmith M J, Cleary P A.et al Fellow eye abnormalities in acute unilateral optic neuritis. Experience of the optic neuritis treatment trial. Ophthalmology 1993100691–697. [DOI] [PubMed] [Google Scholar]