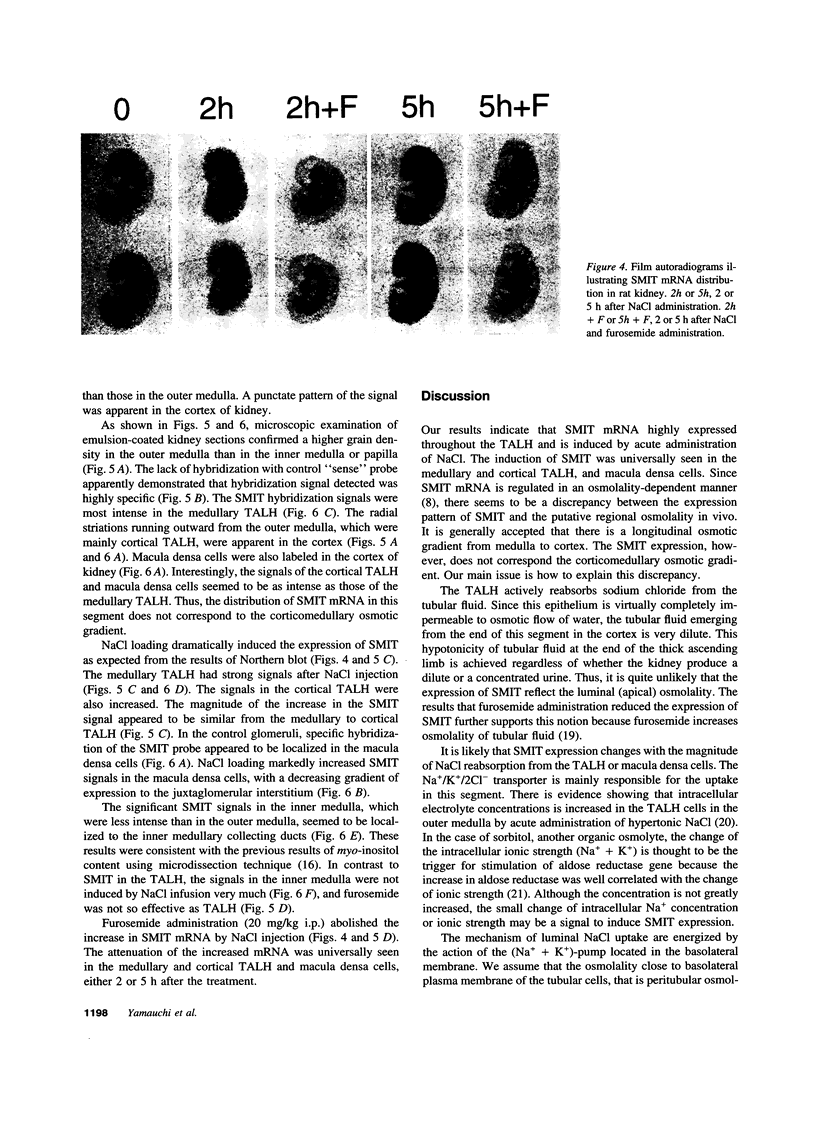
Abstract
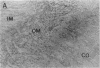
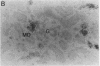
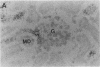
myo-inositol, a major compatible osmolyte in renal medulla, is accumulated in several kinds of cells under hypertonic conditions via Na+/myo-inositol cotransporter (SMIT). To investigate the physiological role of the SMIT, we sought to determine its localization by in situ hybridization and its acute regulation by NaCl and furosemide administration. Northern analysis demonstrated that SMIT is strongly expressed in the medulla and at low levels in the cortex of kidney. Intraperitoneal injection of NaCl rapidly induced SMIT mRNA in both the cortex and medulla, and furosemide completely abolished this induction. In situ hybridization revealed that SMIT it predominantly present in the medullary and cortical thick ascending limbs of Henle's loop (TALH) and macula densa cells. Less intense signals were seen in the inner medullary collecting ducts (IMCD). NaCl loading increased the signals throughout the TALH, and furosemide reduced the signals. SMIT in the IMCD is less sensitive to these kinds of acute regulation. Thus, the distribution pattern of SMIT does not correspond to the corticomedullary osmotic gradient, and SMIT in the TALH and macula densa cells is regulated very rapidly. These results suggest that SMIT expression in TALH may be regulated by intracellular and/or peritubular tonicity close to the basolateral membrane, which is supposed to be proportional to the magnitude of NaCl reabsorption.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagnasco S., Balaban R., Fales H. M., Yang Y. M., Burg M. Predominant osmotically active organic solutes in rat and rabbit renal medullas. J Biol Chem. 1986 May 5;261(13):5872–5877. [PubMed] [Google Scholar]
- Beck F. X., Sone M., Dörge A., Thurau K. Effect of increased distal sodium delivery on organic osmolytes and cell electrolytes in the renal outer medulla. Pflugers Arch. 1992 Dec;422(3):233–238. doi: 10.1007/BF00376207. [DOI] [PubMed] [Google Scholar]
- Chamberlin M. E., Strange K. Anisosmotic cell volume regulation: a comparative view. Am J Physiol. 1989 Aug;257(2 Pt 1):C159–C173. doi: 10.1152/ajpcell.1989.257.2.C159. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Financsek I., Mizumoto K., Muramatsu M. Nucleotide sequence of the transcription initiation region of a rat ribosomal RNA gene. Gene. 1982 May;18(2):115–122. doi: 10.1016/0378-1119(82)90109-3. [DOI] [PubMed] [Google Scholar]
- Garcia-Perez A., Burg M. B. Renal medullary organic osmolytes. Physiol Rev. 1991 Oct;71(4):1081–1115. doi: 10.1152/physrev.1991.71.4.1081. [DOI] [PubMed] [Google Scholar]
- Gullans S. R., Verbalis J. G. Control of brain volume during hyperosmolar and hypoosmolar conditions. Annu Rev Med. 1993;44:289–301. doi: 10.1146/annurev.me.44.020193.001445. [DOI] [PubMed] [Google Scholar]
- Kwon H. M., Yamauchi A., Uchida S., Preston A. S., Garcia-Perez A., Burg M. B., Handler J. S. Cloning of the cDNa for a Na+/myo-inositol cotransporter, a hypertonicity stress protein. J Biol Chem. 1992 Mar 25;267(9):6297–6301. [PubMed] [Google Scholar]
- Miyai A., Yamauchi A., Nakanishi T., Sugita M., Takamitsu Y., Yokoyama K., Itoh T., Andou A., Kamada T., Ueda N. Na+/myo-inositol cotransport is regulated by tonicity in cultured rat mesangial cells. Kidney Int. 1995 Feb;47(2):473–480. doi: 10.1038/ki.1995.60. [DOI] [PubMed] [Google Scholar]
- Nakanishi T., Balaban R. S., Burg M. B. Survey of osmolytes in renal cell lines. Am J Physiol. 1988 Aug;255(2 Pt 1):C181–C191. doi: 10.1152/ajpcell.1988.255.2.C181. [DOI] [PubMed] [Google Scholar]
- Nakanishi T., Turner R. J., Burg M. B. Osmoregulatory changes in myo-inositol transport by renal cells. Proc Natl Acad Sci U S A. 1989 Aug;86(15):6002–6006. doi: 10.1073/pnas.86.15.6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes A., McManus M., Kwon H. M., Strange K. Osmoregulation of Na(+)-inositol cotransporter activity and mRNA levels in brain glial cells. Am J Physiol. 1992 Dec;263(6 Pt 1):C1282–C1288. doi: 10.1152/ajpcell.1992.263.6.C1282. [DOI] [PubMed] [Google Scholar]
- Persson B. E., Sakai T., Marsh D. J. Juxtaglomerular interstitial hypertonicity in Amphiuma: tubular origin-TGF signal. Am J Physiol. 1988 Mar;254(3 Pt 2):F445–F449. doi: 10.1152/ajprenal.1988.254.3.F445. [DOI] [PubMed] [Google Scholar]
- Schmolke M., Bornemann A., Guder W. G. Polyol determination along the rat nephron. Biol Chem Hoppe Seyler. 1990 Oct;371(10):909–916. doi: 10.1515/bchm3.1990.371.2.909. [DOI] [PubMed] [Google Scholar]
- Uchida S., Garcia-Perez A., Murphy H., Burg M. Signal for induction of aldose reductase in renal medullary cells by high external NaCl. Am J Physiol. 1989 Mar;256(3 Pt 1):C614–C620. doi: 10.1152/ajpcell.1989.256.3.C614. [DOI] [PubMed] [Google Scholar]
- Wirthensohn G., Lefrank S., Schmolke M., Guder W. G. Regulation of organic osmolyte concentrations in tubules from rat renal inner medulla. Am J Physiol. 1989 Jan;256(1 Pt 2):F128–F135. doi: 10.1152/ajprenal.1989.256.1.F128. [DOI] [PubMed] [Google Scholar]
- Yamauchi A., Nakanishi T., Takamitsu Y., Sugita M., Imai E., Noguchi T., Fujiwara Y., Kamada T., Ueda N. In vivo osmoregulation of Na/myo-inositol cotransporter mRNA in rat kidney medulla. J Am Soc Nephrol. 1994 Jul;5(1):62–67. doi: 10.1681/ASN.V5162. [DOI] [PubMed] [Google Scholar]
- Yamauchi A., Uchida S., Preston A. S., Kwon H. M., Handler J. S. Hypertonicity stimulates transcription of gene for Na(+)-myo-inositol cotransporter in MDCK cells. Am J Physiol. 1993 Jan;264(1 Pt 2):F20–F23. doi: 10.1152/ajprenal.1993.264.1.F20. [DOI] [PubMed] [Google Scholar]
- Yancey P. H., Clark M. E., Hand S. C., Bowlus R. D., Somero G. N. Living with water stress: evolution of osmolyte systems. Science. 1982 Sep 24;217(4566):1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]
- Zhou C., Agarwal N., Cammarata P. R. Osmoregulatory alterations in myo-inositol uptake by bovine lens epithelial cells. Part 2: Cloning of a 626 bp cDNA portion of a Na+/myo-inositol cotransporter, an osmotic shock protein. Invest Ophthalmol Vis Sci. 1994 Mar;35(3):1236–1242. [PubMed] [Google Scholar]