Abstract
Aims
To determine the effect of glaucomatous damage on the latency of the multifocal visual evoked potential (mfVEP).
Methods
Monocular mfVEPs were recorded from a glaucoma group (n = 50) defined by a glaucomatous disc and an abnormal visual field and a control group (n = 47). 25 patients were characterised as normal tension glaucoma (NTG) and 25 as high tension glaucoma (HTG). Monocular and interocular latency analyses of the more affected eye were obtained using custom software.
Results
On interocular analysis, both the HTG and NTG groups showed a statistically significant increase in mean mfVEP latency with average relative latencies and percentage of points with significant delays of 1.7 ms and 10.3% (HTG) and 1.3 ms and 8.2% (NTG) compared to −0.3 ms and 2.7% (controls). On monocular analysis, only the HTG group showed a significant increase in latency with measures of 5.7 ms and 14.6% (HTG) compared to 3.2 ms and 10.6% (NTG) and 2.1 ms and 9.6% (controls). Using the 95th percentile of a normative group as the cut off, the sensitivity ranged from 20% to 38% and the specificity from 87% to 100% with the interocular analysis providing the best discrimination,
Conclusion
Although up to 40% of patients showed delays in the mfVEP latency, these delays were modest, on average a few milliseconds. These results differ markedly from those of a recent conventional VEP study, which reported 100% sensitivity, 100% specificity, and an average delay that exceeded 25 ms.
Keywords: glaucoma, visual evoked potential, multifocal latency
Glaucoma, a widely prevalent eye disease, is characterised by an optic neuropathy, often associated with elevated intraocular pressure, leading to characteristic visual field defects and optic nerve head damage. It is well established that damage to the ganglion cells and/or their axons produce these visual field defects. What is less clear is the extent to which the ganglion cells undergo a rapid apoptotic death as opposed to lingering in an abnormal state. If the latter holds, then it raises the possibility of neuroprotection of unhealthy retinal ganglion cells. A possible indicator of the health of the retinal ganglion cells is the latency of their response.
There have been a number of studies with conventional visual evoked potentials (cVEP) showing large latency delays, of the order of 20 ms, in glaucoma patients.1,2,3,4,5 A recent study5 reported that all their patients with open angle glaucoma had abnormal cVEP latencies with an average latency that was 27.8 ms longer than the control group. Further, the cVEP latency has been used as a marker of reversible ganglion cell damage in trials of neuroprotective agents for the treatment of glaucoma.6 The implication is that latency can be used as a measure of early glaucomatous damage before retinal ganglion cell death.
A potential problem with the cVEP is that it represents the weighted sum of many local responses. Thus, the technique may obscure delays in local responses. These delays in latency should be seen more easily with the multifocal VEP (mfVEP). With the mfVEP technique, multiple responses, correlating with specific localised regions of the visual field, can be tested simultaneously.7 A number of studies have shown the relatively high sensitivity of the mfVEP in detecting glaucomatous damage.8,9,10,11,12,13,14,15,16,17 However, nearly all of this work has been based on amplitude measures of the mfVEP. Relatively little has been reported about the latency of mfVEP responses in glaucoma patients. A report, in abstract form, suggested rather small latency changes with glaucomatous damage.18 Considering the implications for neuroprotection, a greater understanding of mfVEP latency in patients with glaucoma is important. Here we investigate the effect of glaucoma on the latency of the mfVEP responses.
Methods
Subjects
High tension glaucoma
This group consisted of 25 patients with intraocular pressures > 21 mm Hg. The inclusion criteria were (1) cup to disc ratio > 0.6, (2) abnormal glaucoma hemifield test (GHT) on 24–2 Humphrey visual field (HVF), (3) open angles, (4) a mean deviation (MD) on HVF of better than −8 dB in both eyes. The patients' age ranged from 30 years to 77 years with a mean age of 58 (SD12) years. The average MD was −4.2 (SD 1.7) dB and on average the more affected eye had an MD that was 2.9 (SD 1.9) dB lower than the less affected eye.
Normal tension glaucoma
This group consisted of 25 patients with IOP <21 mm Hg but otherwise satisfying the same criteria as the HTG group. The patients' age ranged from 34 years to 75 years with a mean age of 59.6 (SD 12) years. The average MD was −3.6 (SD 2.2) dB and on average the more affected eye had a MD that was 2.1 (SD 1.9) dB lower than the less affected eye.
Controls
This group consisted of 47 individuals with normal vision who ranged in age from 31 years to 81 years with a mean age of 50 (SD 10) years. These individuals had a visual acuity ⩾ 20/20, a normal fundus examination, and a normal HVF (that is, normal GHT and MD). The average MD was −0.8 (SD 1.1) dB.
Procedures followed the tenets of the Declaration of Helsinki, and the protocol was approved by the committee of the institutional board of research of Columbia University.
mfVEP stimulus
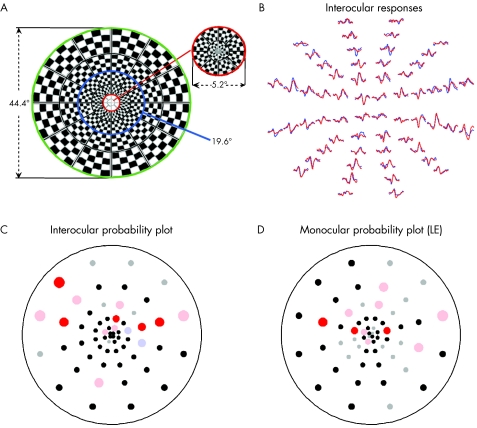
The dartboard display (fig 1A), viewed through natural pupils with the appropriate refractive correction, consisted of 60 sectors, each with 16 checks, eight white (200 cd/m2) and eight black (<1 cd/m2). Each of the 60 sectors followed an independent pseudorandom sequence of pattern reversals in which there was a 50% probability of reversing at each frame shift. For details about the mfVEP see Baseler et al7 and Hood and Greenstein.14
Figure 1 (A) The mfVEP stimulus display of 60 cortically scaled sectors. Each of the 60 sectors of the display is an independent pattern reversing checkerboard. (B) Sample responses for a patient with glaucoma for monocular stimulation of the left (red) and right (blue) eyes. (C) Latency probability plots for the interocular analysis of the responses in (B). (D) Latency probability plots for the monocular analysis of the responses from the left eye in (B).
mfVEP recording
Three signal channels were recorded simultaneously with gold cup electrodes using Veris 4.3 software (Electro‐Diagnostic Imaging, San Mateo, CA, USA). The ground and reference electrodes were placed on the forehead and inion, respectively. The three active electrodes were placed 4 cm above the inion, and 4 cm on either side of, and 1 cm above, the inion. All three channels were filtered with a high and low frequency cutoff of 3 Hz and 100 Hz (Grass Instruments preamplifier P511J, Quincy, MA, USA). The resistance was less than 5 k for all subjects. Four monocular, 7 minute recordings were obtained, two for each eye (ABBA order). See Hood and Greenstein14 and Hood et al19 for more details.
mfVEP analysis
The exported mfVEP records were processed using custom software written in MATLAB (Mathworks Inc, Natick, MA, USA) and an array of best channel responses derived based upon signal to noise ratio as previously described.14,19,20 The mfVEP best channel responses for a NTG patient are shown as the records in colour in figure 1B.
Relative monocular latency and the interocular difference in latency were determined at each of the 60 locations and compared with a normative set using computerised techniques previously described.21,22 Briefly, relative monocular latencies were determined by shifting the subject's best channel response along the time axis to give the maximal overlap (cross correlation) with a template trace determined from a normative group of 100 individuals with a mean age of 49 (SD 13.6) ms.23 The amount of shift was the relative monocular latency, compared to the norms, in milliseconds. Only the more affected eye, based on 24–2 Humphrey visual field (HVF) mean deviation, was included in the monocular latency analysis. The difference in interocular latencies at each location was determined by shifting the right eye response along the time axis for best cross correlation with the left eye. The amount of shift was the interocular latency difference, with a positive value signifying that the response of the more affected eye was slower than that of the less affected eye.
The control and the normative groups were, on average, nearly 10 years younger than the patient groups. However, the effects of age are relatively small (1.3 ms/decade) for the monocular latency measures and very small (0.1 ms/decade) for the interocular latency measures.21,22 In addition, this suggests that the relatively small latency increases we find for the patients might be slightly smaller if age were taken into consideration.
Probability plots were created by comparing the latency measures to those of the normative set. Interocular and monocular latency probability plots for a typical NTG patient are shown in figures 1C and D. The points on the plot are located at the centres of the 60 sectors in the mfVEP display. Blue (right eye) and red (left eye) circles represent locations that are delayed at either the 5% (desaturated) or 1% (saturated) level. Grey circles represent responses that are either too small (signal to noise ratio <1.7) or with waveforms deviating too much from normal templates (cross correlation < 0) to give reliable latency values. See Hood et al21,22 for details.
Results
Examples of mfVEP responses and latency probability plots for a typical NTG patient are shown in figure 1. Figure 1B shows the mfVEP responses from both eyes (red: LE; blue: RE). As indicated by the coloured circles in figures 1C and D, 14 and 10 locations were significantly delayed on the interocular and monocular probability plots, respectively. However, these delays were relatively small. As will be seen below, the results for this patient were typical.
Average latency
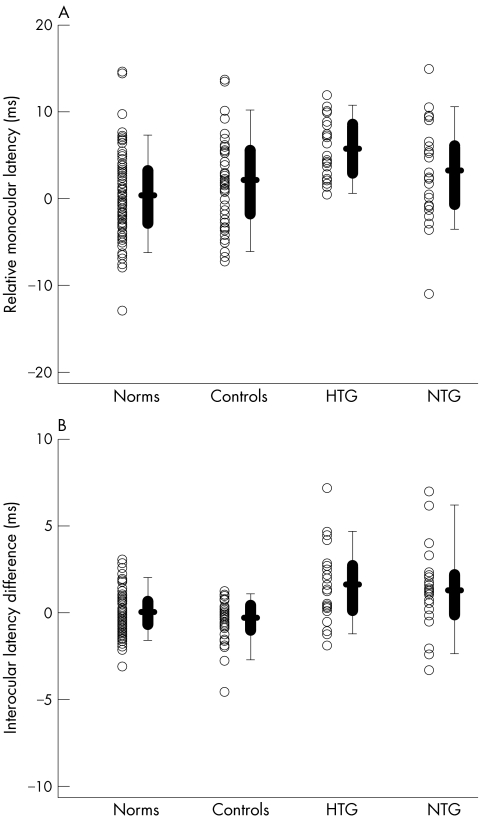
The monocular analysis provides the most direct comparison to previous cVEP data. Each symbol in figure 2A is the average relative monocular latency for an individual. An individual's relative monocular latency is the average relative monocular latency of all traces meeting reliability criteria for the more affected eye (poorer MD on HVF). It is clear from figure 2A that there is considerable overlap among the groups. This is easier to see in the box plots. In this presentation, the 25% and 75% range and the 5% and 95% range are shown by the box and lines, respectively, with the bold horizontal bar indicating the mean. Table 1 contains the means for the different groups where, for example, a value of 3 ms indicates that, on average, this group's mfVEPs were delayed by 3 ms relative to the normative group. Only the HTG group showed a significant difference from controls with a mean value of 5.7 ms (Mann‐Whitney rank sum test; p<0.001) compared to 3.2 ms for NTG and 2.1 ms for controls. Only one patient (1 NTG) fell above the range of control values, while 30% (15 patients: 10 HTG and 5 NTG) fell above the 95th percentile for the norms. Using the 95th percentile of the norms as a criterion, the sensitivity and specificity were 30% and 87%, respectively (table 2).
Figure 2 (A) Average monocular latency of the more affected eye (poorer MD) relative to a normative group is shown for individual eyes (symbols) of the normative (n = 100), control (n = 47), NTG (n = 25), and HTG (n = 25) groups. The box plot shows the 25/75% range (box), the 5/95% range (vertical line) and the mean of the group (horizontal bar). (B) As in (A) for the average interocular latency difference for each individual.
Table 1 Mean (median) latency and percentage of points delayed for both monocular and interocular tests.
| Group | Mean relative monocular latency (ms) | Mean interocular latency difference (ms) | % Monocular points delayed | % Interocular points delayed |
|---|---|---|---|---|
| Normative | 0.4 | 0.1 | 5.8% | 2.7% |
| Control | 2.1 | −0.3 | 9.6% | 2.7% |
| HTG | 5.7** | 1.7** | 14.6%* | 10.3%** |
| NTG | 3.2 | 1.3** | 10.6% | 8.2%** |
*p<0.02 when compared to the control group.
**p<0.001 when compared to the control group.
Table 2 Specificity and sensitivity for monocular and interocular latency and percentage of points criteria.
| Group | Mean relative monocular latency (ms) | Mean interocular latency difference (ms) | % Monocular points delayed | % Interocular points delayed |
|---|---|---|---|---|
| Specificity (controls) | 87% | 100% | 91% | 98% |
| Sensitivity (NTG + HTG) | 30% | 36% | 20% | 38% |
The interocular analysis does a slightly better job of distinguishing among the patients and the controls. Each symbol in figure 2B is the average interocular latency. An individual's average interocular latency difference was calculated as the mean of the interocular latency differences for all locations meeting reliability criteria. Positive values signify the eye with the poorer MD was delayed relative to the eye with the better MD. The box plots are described above. Although there is overlap among individual values and the interocular differences were relatively small, both the HTG and NTG groups showed significantly higher mean interocular latency values of 1.7 ms and 1.3 ms (Mann‐Whitney rank sum test; p<0.001), respectively, compared to −0.3 ms for controls. Fifty per cent (25 patients: 13 HTG and 12 NTG) of the patients fell above the range of control values and 36% (20 patients: 10 HTG and eight NTG) fell above the 95th percentile for the norms. The sensitivity and specificity were 36% and 100%, respectively.
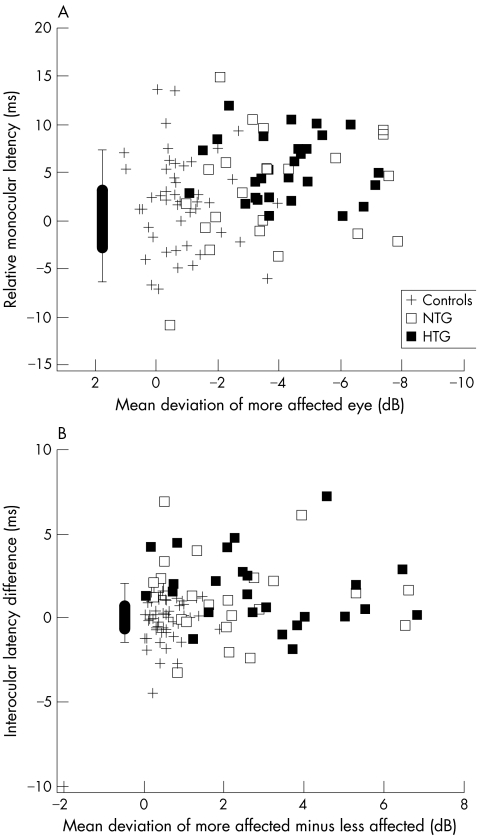
The latency data from figure 2 for the three groups of subjects are shown as a function of MD in figure 3. For the monocular data (fig 3A) the latency and MD values are for the more affected eye, while for the interocular data (fig 3B) the latency is plotted against the difference between the MDs of the more (poorer MD) and less affected eyes. There was no relation between either the monocular (fig 3A) or interocular latency (fig 3B) and MD.
Figure 3 (A) Average relative monocular latency of the more affected eye (poorer MD) as a function of the mean deviation of that eye is shown for individual eyes (symbols) of the control (+), NTG (open square), and HTG (solid square) groups. The box plot shows the 25%/75% range (box), the 5%/95% range (vertical line) and the mean of the normative group (horizontal bar). Its placement along the x axis is arbitrary. (B) As in (A) for the average interocular latency difference.
Percentage delayed traces
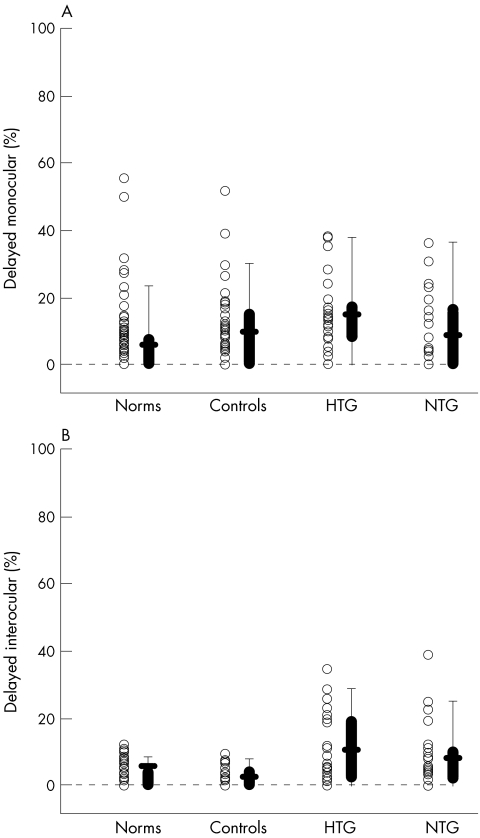
To take advantage of the localising ability of the mfVEP, the latency probability plots (fig 1C, D) were analysed. For each subject the percentage of significantly delayed responses was determined by dividing the number of significant (coloured) locations in figure 1C and D by the total number of responses that met criteria for measurement (that is, 60 minus the number of grey locations). Table 1 shows the mean results. For the interocular analysis, both the HTG and NTG groups differed significantly from controls with a mean of 10.3% and 8.2% significantly delayed responses compared to 2.7% for controls (p<0.001). Only the HTG group was significantly different on monocular analysis with 14.6% delayed (p = 0.013) compared to 10.6% for NTG and 9.6% for controls.
The symbols in figure 4 are each individual's percentage of locations delayed and the box plots are as described above. For the monocular analysis (fig 4A), there is considerable overlap of the groups. Only 2% (one NTG) of the patients fell above the range of control values, while 20% (10 patients: five NTG and five HTG) fell above the 95th percentile for the norms. Using the 95th percentile as a criterion, the sensitivity and specificity were 20% and 91%, respectively (table 2). For the interocular analysis, 32% (16 patients: nine HTG and seven NTG) fell above the range of control values and 38% (19 patients: 11 HTG and eight NTG) were above the 95th percentile for the norms. The sensitivity and specificity were 38% and 98%, respectively.
Figure 4 (A) The percentage of points in the more affected eye (poorer MD) with significant delays (see fig 1B) is shown for individual eyes (symbols) of the normative (n = 100), control (n = 47), NTG (n = 25), and HTG (n = 25) groups. The box plot shows the 25/75% range (box), the 5/95% range (vertical line) and the mean of the group (horizontal bar). (B) As in panel A for the percentage of points in the interocular probability plot.
Discussion
The identification of glaucoma patients with abnormal latencies could open the possibility of neuroprotection of unhealthy retinal ganglion cells. In this regard, reports of substantial delays in the conventional VEP (cVEP) encouraged us to assess delays with the locally more sensitive mfVEP technique. The delays in the glaucoma group were modest, on average less than 4 ms, when compared to the control group, and involved fewer than 40% of the patients.
Our results provide a marked contrast to those recently reported by Parisi et al.5 In that study, all 84 patients with open angle glaucoma had cVEP latencies that were longer than the longest latency found among the 80 normal control subjects. Further, the mean latency of the OAG group was 27.8 ms longer than that of the control group. The monocular mean latency analysis of our HTG group provides the most direct comparison to their study. Our HTG group had, on average, an increase in latency of only 5.3 ms compared to the normative group and only 3.6 ms compared to the control group. Further, there was considerable overlap with the control and normative groups with only one patient's value falling above the control group range. Using the 95th percentile of the normative data as a definition of abnormal latency resulted in a sensitivity and specificity of 30% and 87%, respectively, values far from the 100% sensitivity and specificity reported for the cVEP by Parisi et al.5
For our patients, the interocular test for mean latency does a little better. Using the 95th percentile of the norms, the sensitivity and specificity were 36% and 100%, respectively. Using the percentage of points in the field with abnormal latencies yielded similar results with again the interocular comparison providing better discrimination than the monocular comparison.
The reasons for these discrepancies compared with the study of Parisi et al are not entirely clear. While the OAG patients in their study had, on average, more severe field losses than our HTG group, their results were substantially the same for their patients with MDs in the same range (better than −8 dB) as ours. In addition, other patient characteristics (for example, age) cannot explain the difference in results. On the other hand, the mfVEP and cVEP techniques differ in both the stimulus used and the analysis employed. Further, the evidence suggests that the mfVEP has less of a post V1 contribution, than does the cVEP. Theoretically it is possible the delays are introduced beyond V1. However, before invoking such speculative explanations, recordings of both cVEP and mfVEP from the same group of patients need to be made. We are completing such a study.
In summary, in a group of patients with glaucoma and mild to moderate visual field loss, the delays in the mfVEP were modest. On average the delays were a few milliseconds and they rarely exceeded 10 ms. On the other hand, up to 40% of these patients may have abnormal latencies and these are best detected with an interocular analysis. Before a decision is made to use either the mfVEP or cVEP in neuroprotection trials the discrepancy between the mfVEP and cVEP needs to be understood.
Abbreviations
cVEP - conventional visual evoked potentials
GHT - glaucoma hemifield test
HTG - high tension glaucoma
HVF - Humphrey visual field
MD - mean deviation
mfVEP - multifocal visual evoked potential
NTG - normal tension glaucoma
VEP - visual evoked potential
Footnotes
Supported by grant EY02115 from the National Institutes of Health, Bethesda, MD and by the Shelley and Steven Einhorn Research Fund of the New York Glaucoma Research Institute, New York, USA.
A preliminary version of this work was presented at the meeting of ARVO in May 2005.
References
- 1.Atkin A, Bodis‐Wollner I, Podos S M.et al Flicker threshold and pattern VEP latency in ocular hypertension and glaucoma. Invest Ophthalmol Vis Sci 1983241524–1528. [PubMed] [Google Scholar]
- 2.Towle V L, Moskowitz A, Sokol S.et al The visual evoked potential in glaucoma and ocular hypertension: effects of check size, field size, and stimulation rate. Invest Ophthalmol Vis Sci 198324175–183. [PubMed] [Google Scholar]
- 3.Parisi V. Neural conduction in the visual pathways in ocular hypertension and glaucoma. Graefes Arch Clin Exp Ophthalmol 1997235136–142. [DOI] [PubMed] [Google Scholar]
- 4.Parisi V. Impaired visual function in glaucoma. Clinical Neurophysiol 2001112351–358. [DOI] [PubMed] [Google Scholar]
- 5.Parisi V, Miglior S, Manni G.et al Clinical ability of pattern electroretinograms and visual evoked potentials in detecting visual dysfunction in ocular hypertension and glaucoma. Ophthalmology 2006113216–228. [DOI] [PubMed] [Google Scholar]
- 6.Rejdak R, Toczolowski J, Krukowski J.et al Oral citicoline treatment improves visual pathway function in glaucoma. Med Sci Monit 20039P124. [PubMed] [Google Scholar]
- 7.Baseler H A, Sutter E E, Klein S A.et al The topography of visual evoked response properties across the visual field. Electroenceph Clin Neurophysiol 19949065–81. [DOI] [PubMed] [Google Scholar]
- 8.Klistorner A I, Graham S L, Grigg J R.et al Multifocal topographic visual evoked potential: improving objective detection of local visual field defects. Invest Ophthalmol Vis Sci 199839937–950. [PubMed] [Google Scholar]
- 9.Hood D C, Zhang X, Greenstein V C.et al An interocular comparison of the mul‐tifocal VEP: a possible technique for detecting local damage to the optic nerve. Invest Ophthalmol Vis Sci 2000411580–1587. [PubMed] [Google Scholar]
- 10.Graham S L, Klistorner A I, Grigg J R.et al Objective VEP perimetry in glaucoma: asymmetry analysis to identify early deficits. J Glaucoma 2000910–19. [DOI] [PubMed] [Google Scholar]
- 11.Hasegawa S, Abe H. Mapping of glaucomatous visual field defects by multifocal VEPs. Invest Ophthalmol Vis Sci 2001423341–3348. [PubMed] [Google Scholar]
- 12.Goldberg I, Graham S L, Klistorner A I. Multifocal objective perimetry in the detection of glaucomatous field loss. Am J Ophthalmol 200213329–39. [DOI] [PubMed] [Google Scholar]
- 13.Fortune B, Goh K, Demirel S.et al Detection of glaucomatous field loss using Multifocal VEP. In: Perimetry update 2002/2003. The Hague, Netherlands: Kugler Publications, 2004251–260.
- 14.Hood D C, Greenstein V C. Multifocal VEP and ganglion cell damage: applications and limitations for the study of glaucoma. Prog Retin Eye Res 200322201–251. [DOI] [PubMed] [Google Scholar]
- 15.Thienprasiddhi P, Greenstein V C, Chen C S.et al Multifocal VEP responses in glaucoma patients with unilateral hemifield defects. Am J Ophthalmol 200313634–40. [DOI] [PubMed] [Google Scholar]
- 16.Hood D C, Thienprasiddhi P, Greenstein V C.et al Detecting early to mild glaucomatous damage: a comparison of the multifocal VEP and automated perimetry. Invest Ophthalmol Vis Sci 200445492–498. [DOI] [PubMed] [Google Scholar]
- 17.Graham S L, Klistorner A I, Goldberg I. Clinical application of objective perimetry using multifocal visual evoked potentials in glaucoma practice. Arch Ophthalmol 2005123729–739. [DOI] [PubMed] [Google Scholar]
- 18.Klistorner A, Balachandran C, Graham S L.et al Multifocal VEP latency in glaucoma [ARVO abstract]. Invest Ophthalmol Vis Sci. 2002;43: E‐abstract, 2165
- 19.Hood D C, Zhang X, Hong J E, Chen C S. Quantifying the benefits of additional channels of multifocal VEP recording. Doc Ophthalmol 2002104303–320. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Hood D C, Chen C S.et al A signal‐to‐noise analysis of multifocal VEP responses: an objective definition for poor records. Doc Ophthalmol 2002104287–302. [DOI] [PubMed] [Google Scholar]
- 21.Hood D C, Ohri N, Yang E B.et al Determining abnormal latencies of multifocal visual evoked potentials: A monocular analysis. Doc Ophthalmol 2004109189–199. [DOI] [PubMed] [Google Scholar]
- 22.Hood D C, Zhang X, Rodarte C.et al Determining abnormal interocular latencies of multifocal visual evoked potentials. Doc Ophthalmol 2004109177–187. [DOI] [PubMed] [Google Scholar]
- 23.Fortune B, Zhang X, Hood D C.et al Normative ranges and specificity of the multifocal VEP. Doc Ophthalmol 200410987–100. [DOI] [PubMed] [Google Scholar]