Abstract
Background
In age related macular degeneration and inherited dystrophies, preservation of retinal ganglion cells has been demonstrated. This finding has led to the development of various models of subretinal or epiretinal implant in order to restore vision. This study addresses the development of a polyimide subretinal electrode platform in the dystrophic P23H rat in vivo.
Methods
A technique was developed for implanting a subretinal electrode into the subretinal space and stabilising the distal extremity of the cabling on the rat cranium in order to allow future electrical stimulations of the retina.
Results
In vivo imaging of the retina with the scanning laser ophthalmoscope demonstrated reabsorption of the surgically induced retinal detachment and the absence of major tissue reactions. These in vivo observations were confirmed by retinal histology. The extraocular fixation system on the rat cranium was effective in stabilising the distal connector for in vivo stimulation.
Conclusion
This study demonstrates that a retinal implant can be introduced into the subretinal space of a dystrophic rat with a stable external connection for repeatable electrical measurements and stimulation. This in vivo model should therefore allow us to evaluate the safety and efficacy of electrical stimulations on dystrophic retina.
Keywords: photoreceptor degeneration, retina, prosthesis, impedance, rat
In retinal dystrophies and age related macular degeneration (AMD), visual loss occurs as a consequence of photoreceptor degeneration. As a result, a secondary process of partial trans‐synaptic degeneration occurs affecting neurons distal to the degenerating photoreceptors. There is histological evidence of some degree of ganglion cell sparing.1,2,3,4,5 Humayun and co‐workers have reported that in vivo electrical stimulation of surviving retinal ganglion cells was able to generate phosphenes correlated spatially and temporally with the stimuli, demonstrating that these neurons were still able to convey visual information to the brain.6,7 These findings support the assumption that electrical stimulation of surviving retinal cells by a retinal prosthesis might restore useful vision in AMD or retinal dystrophy. Strategies that have been proposed in order to trigger coordinated ganglion cell activity include subretinal stimulation,8,9,10 epiretinal stimulation on the retinal inner limiting membrane,11,12,13,14,15,16 or perineural stimulation around the optic nerve.17,18
The efficacy and safety of retinal implants have been investigated in different animal species in vivo. A number of critical issues remain outstanding, such as surgical techniques, stimulation protocols, implant tolerance, and biocompatibility.
Subretinal prostheses have been implanted in living wild type pigs and cats where photoreceptors facing the implants eventually underwent degeneration associated with glial tissue formation.19 The retinal response to light was not, however, significantly altered by the presence of the implant as indicated by electroretinograph recordings. Retinal stimulation by the subretinal implant also elicited visual evoked potentials consistent with an activation of retinal ganglion cells.20,21,22 Self powered retinal implants have been introduced into the subretinal space of RCS rats, an animal model of retinitis pigmentosa, in order to demonstrate that the subretinal implant could slow the congenital retinal degeneration.23 This photoreceptor rescue was ascribed to a trophic effect of the implant on the retina. A similar effect was seen in human experiments where visual performance improved at sites distant from the implant itself.24
The aim of this study was to design a surgical approach in vivo in the rat eye using a subretinal implant activated by an external connector in order to evaluate the efficacy and safety of the stimuli on the retina. The dystrophic P23H rat was selected for these experiments because this animal model of retinitis pigmentosa may provide a similar biological environment to patients with inherited photoreceptor degeneration. The study shows that subretinal implants can be inserted for long term studies.
Materials and methods
Implants
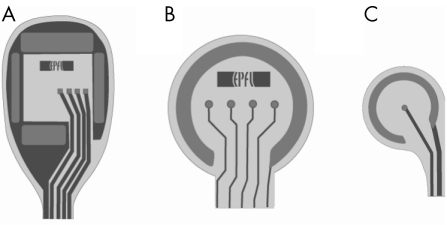
Initial experiments were conducted with inert polyimide (type PI2611) prototypes in order to test the technique and feasibility of the procedure. In later experiments, we implanted devices with one or several platinum electrodes with a 25 μm radius (fig 1). These were produced at the Laboratory of Microsystems, EPFL, using a standard procedure.25 In brief, a layer of 11 μm polyimide (PI12611, HD Microsystems) was applied by spin coating, and subsequently cured. A 200 nm thick layer of platinum was deposited on the polyimide, using titanium as the adhesion layer. The electrodes were shaped using dry etching. A second layer of polyimide was then deposited, and the implant shape and electrode openings were engineered by dry etching. The final total implant thickness was 22 μm. The electrodes were then soaked in 10% HCl and calibrated using Cole‐Palmer traceable test solutions. To allow diffusion of nutrients and waste products, some implants were also supplied with through holes with a diameter of 25 μm.
Figure 1 Different retinal implant designs. (A) A 3 mm wide device with four central stimulating electrodes and a reference electrode on the four sides. (B) A 1 mm device with four stimulating electrodes and a large circular reference electrode. Some of these were equipped with 25 μm holes for increased diffusion. (C) A 250 μm asymmetric implant with only one central electrode.
Animals
Animal experiments were conducted in accordance with the European Communities Council Directive (86/609/EEC) and the Association for Research in Vision and Ophthalmology (ARVO statement on the use of animals in ophthalmic and vision research). Animals were maintained on a normal diet under a 12 hour light/dark cycle. In all cases, surgery was performed in animals of at least 3 months. After pupil dilatation with a drop of tropicamide (2 mg/0.4 ml, Ciba Vision Ophthalmics, Blagnac, France), P23H transgenic rats (line 1) were anaesthetised by intramuscular injection with a 4:1 mixture of ketamine‐xylazine (ketamine 100 mg/kg, xylazine 10 mg/kg) (Ketamine 500: Virbac, Carros, France; xylazine 2%: Rompun, Bayer Pharma, Puteaux, France). One eye of each animal was implanted.
Implantation techniques
Once the animal was anaesthetised, topical oxybuprocaine chlorhydrate 1.6 mg/0.4 ml (Novartis Pharma, 92500 Rueil‐Malmaison, France) was instilled on the operated side. A superior conjunctival peritomy was performed. A small radial sclerotomy was made 1.5 mm behind the limbus with a 25 gauge needle. Biolon (1% sodium hyaluronate, Bio‐Technology General, Rehovot, Israel) was injected into the subretinal space through the sclerotomy in order to obtain a localised retinal detachment visible as a clear “flush wave” posterior to the injection. The polyimide implant was gently inserted anterior to posterior into the subretinal space. The implant “tail” was sewn to the sclera with 10‐0 Nylon. Visual control was obtained with indirect ophthalmoscopy.
In vivo imaging
Scanning laser ophthalmoscope (SLO) images were obtained in four rats (Heidelberg Engineering, Heidelberg, Germany). Fluorescein angiograms were obtained by an intraperitoneal injection of fluorescein (0.3 ml of 4% fluorescein sodium, Fluka Chemie, Buchs, Switzerland). Imaging was obtained without anaesthetising the animals.
Impedance spectroscopy
Impedance spectra were measured with an LCR meter (Agilent 4284A, Palo Alto, CA, USA) controlled by a proprietary Java software. Measurements were taken between one stimulating electrode and the reference circular electrode. Each spectrum consisted of 60 points logarithmically spaced between 100 Hz and 1 MHz, with the sweep starting at the highest frequency.
Histology
At 9 weeks animals were anaesthetised as described above to allow an intracardiac perfusion of 4% paraformaldehyde in phosphate buffer 0.1 M (pH 7.4) at a 10 ml/min rate after clamping blood vessels irrigating the inferior part of the body. Once the eye was enucleated, the cornea and lens were removed by circumferential incision anterior to the ora serrata. The implant tail was sectioned in order to maintain the implant head in the subretinal space. Eye cups were incubated overnight at 4°C in phosphate buffer (0,1 M; pH 7.4) containing 4% paraformaldehyde and 2.5% glutaraldehyde. After several washes in phosphate buffer, the tissue was postfixed in osmium tetroxide 1% for 30 minutes at room temperature and rapidly rinsed in water. Finally, the tissue was dehydrated in alcohol and embedded in epoxy resin. Semithin sections were obtained by ultramicrotome sectioning (Ultracut, Leica) with a histo‐diamond (45°, 8 mm diatome), and stained with toluidine blue.
Results
Implantation and imaging
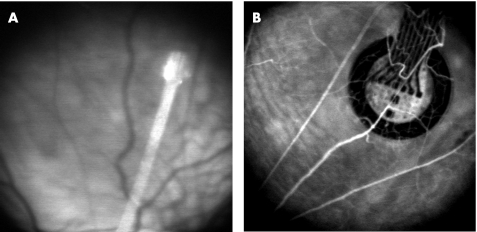
Figure 2 shows two different subretinal implants observed in vivo in the fundus. The first implant prototypes were polyimide (type PI2611) bases only to assess the feasibility of the surgical procedure using various head shapes. Implant head diameters greater than 1 mm required large circumferential sclerotomies. They were difficult to adapt to the tight curvature of the eye. Prototypes with a smaller head diameter were also used but were abandoned as they did not provide sufficient space for multiple channel electrodes. Symmetrical head designs were easier to insert under the retina and did not undergo lateral slippage during insertion as was the case for asymmetrical designs. The most suitable prototype had a symmetrical head design with a 1000 μm diameter size and a connecting tail 250 μm large and 4 cm long (fig 2B). These prototypes were 22 μm thick.
Figure 2 In vivo SLO images of retinal implants in dystrophic rats. (A) Rat fundus showing a 250 μm implant head in the subretinal space. (B) Fluorescein angiography obtained 1.5 months after the surgery and showing retinal blood vessels passing above the subretinal 1000 μm implant head.
During this process, 26 rats underwent subretinal implantation in five operating sessions. Postoperative examinations confirmed that the implants were in the subretinal space when retinal vessels were seen passing over the device. Fourteen of 26 implants were satisfactorily positioned in the subretinal space (54%); the success rate in the last 10 operated animals was 70%. A number of post‐implantation extrusions reduced the very high initial success rate for subretinal space positioning.
After up to 1.5 months, scanning laser ophthalmoscopy (SLO) imaging of the retinas showed no fibrous reaction in the vicinity of the implant and macroscopically normal tissue (fig 2). In one case, however, a haemorrhagic retinal detachment was observed at the level of the implant head.
After establishing the most appropriate shape for the implant we then investigated how an external connector at the distal end of the implant could be made reliably accessible for repeated stimulations and recordings on the living animal. This external connector (six male pins) connected to the implant tail was glued onto a Plexiglas plate with araldite. Implantation was performed under general anaesthesia. A coetaneous incision on the cranial apex was performed and two small bore screws inserted into the cranial apex as previously described for the stabilisation of intracerebral injection cannulas.26 A subcutaneous track was made between the lateral superior fornix of the implanted eye and the skin incision with a 16 gauge cannula. The implant head was “fed” through this track distal to proximal before being inserted under the retina. Once the intraocular procedure was completed, the proximal electrical connector plate was fixed with dental cement to the bore screws in the skull.
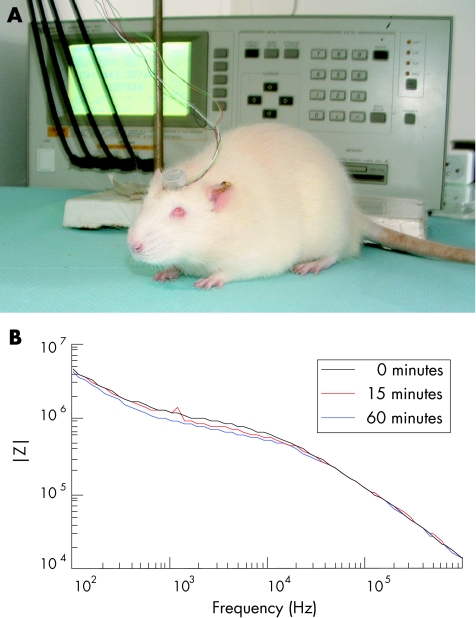
The rapid curing of the paste provided a stable resting platform for the connector. In order to safeguard it from post‐surgical damage, the connector was protected in a casing made out of the proximal end of a screw top Eppendorf tube. To assess whether stimulations can be applied on non‐anesthetised rats, sequential impedance measurements were achieved 1 week after implantation by applying a 50 mV sine wave at different frequencies. In figure 3A, wires are seen leaving the implant connector on the non‐anaesthetised rat head and linking up with an LCR meter (seen in the background) via four BNC cables. Figure 3B illustrates the stability of the impedance measurements at 0, 15, and 60 minutes.
Figure 3 In vivo stimulation for implant impedance measurements. (A) Non‐anaesthetised animal connected to an LCR meter. The Eppendorf cap protecting the external implant connector on the skull is visible. This connector is wired to an LCR meter seen in the background through a switch board (not shown) and four BNC cables. (B) Impedance spectra. Impedance measurement obtained at different frequencies with a 50 mV sine wave stimulation. The measurements were repeated at the beginning of the experiment (0 minutes, dark 15 minutes (red), and 60 minutes (blue)).
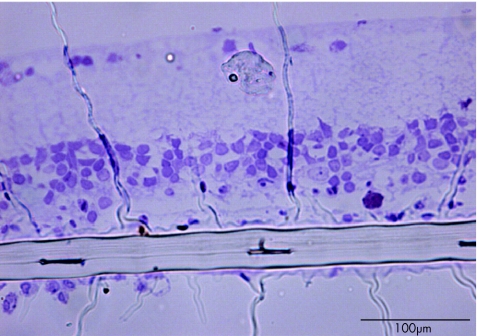
Retinal semi‐thin sections confirmed that the retinal tissue was not greatly altered in the vicinity of the implant (fig 4). On average, ganglion cells were observed at a distance of 149.9 μm (SEM 25.7 μm, n = 5) from the implant below the retina.
Figure 4 Retinal semi‐thin section showing the subretinal implant. Note that retinal cells are in apposition to the retinal implant. The scale bar represents 100 µm.
Discussion
This study demonstrated that it was possible to implant platinum electrodes into the subretinal space of the P23H rat and to maintain a stable electrical connection between the electrodes and the extraocular connector, notwithstanding mechanical stress on the connector cable. The implants remained in the subretinal space for up to 1.5 months without histological evidence of glial scarring. The few cases of proliferative vitreoretinopathy were attributed to the surgeon's learning curve.
Subretinal implantation of various implant designs in mammals such as the cat, the rabbit , the non‐dystrophic laboratory swine and the RCS rat have previously been reported.24,27 In the latter, the subretinal implant was self powered and no external cabling was required.24 Implantation in the rat, pig, and cat has been obtained by elevating the retina with a subretinal fluid injection through a sclerotomy to create an iatrogenic retinal detachment.21,22,24,27,28 The implantation method is simple and reproducible. Subretinal access is achieved by sclerotomy or sclerectomy without entering the vitreous cavity (ab externo) or by retinotomy or limbal sclerotomy using a vitrectomy approach (ab interno). The latter techniques have been used in rabbits,9 minipigs,29 and cats.30 Given the size of the rat globe and lens, this approach is not possible with current instrumentation. Zrenner and co‐workers developed a custom made implantation tool for multiphotodiode array (MPDA) implantation in the rat subretinal space.31 However, these MPDAs were stand alone devices without the extraocular wire connexion used in our experiments. Subretinal implants have also been introduced in dystrophic RCS rats in order to demonstrate the neuroprotective effects of implantation, which were noted with both active and inactive implants.24 These trophic effects, akin to those reported after phosphate buffer injection into the subretinal space,32 were ascribed to the effect of the surgery itself on the retina.
RCS rats have defective phagocytosis of photoreceptor outer segments by the retinal pigment epithelium (RPE). This results from a recessively transmitted Mertk mutation which is solely expressed in the RPE.33 In this study we used P23H transgenic rats with the opsin mutation responsible for the most common form of autosomal dominant retinitis pigmentosa (adRP). Line 1 rats, which were used in these experiments, have the highest level of transgene expression and the fastest rate of photoreceptor degeneration. Histopathological and electrophysiological studies have shown broad similarities between human adRP P23H mutation and P23H rat retinal degeneration.34
The difference in activation threshold between normal and dystrophic human retina that was reported with epiretinal implants further supports the use of a dystrophic mammal model to evaluate stimulating electrodes and protocols. Although surgery in the rat is difficult given the small globe and large lens,35 dystrophic rats, with a lifespan of up to 2 years, provide a unique model in which long term stimulation of the retina can be performed. Electrophysiological measurements were recently achieved in vivo on rats just after implantation but were not performed following chronic stimulations.36 In further studies on P23H rats, which are readily available at a low cost relative to cats or pigs, we plan to investigate the safety and efficacy of long term retinal stimulation using the electrical connector attached to the animal's head as shown in figure 2 as well as changes in electrical impedance over time using electrical impedance spectroscopy, a technique with which it is possible to simultaneously characterise the conductivity of a tissue sample and the surface properties of the electrodes.37 The distance between implant and the retinal tissue as observed on histological sections already suggests that appropriate electrical stimulations of retinal cells should produce activation of visual centres.
Various electrode sizes have been implanted into the subretinal space: eight platinum electrodes of 100 μm×100 μm for direct electrical stimulation of the retina in the pig and rabbit,27 an array of up to 5000 photodiodes with 20 μm×20 μm electrodes powered by incident light in the cat,28 a 2 mm diameter, 25 μm thick chip with approximately 5000 independent microphotodiodes in human volunteers.23
Our prototype has similar sized electrodes (25 μm×25 μm), as we aim to produce a retinal implant with 100 μm square pixel size stimuli. Our group recently reported that a 3.5°×10° “window” containing 286 pixels with a 100 μm square size was sufficient to allow reading of four letter words at 15° of eccentricity and that a window enlarged to 7°×10° (572 pixels) allowed fluent text reading.38,39,40 Based on these results, we designed stimulating test heads with four circular 25 μm radius electrodes and a centre to centre spacing of 150 μm to allow for the generation of pixels larger than 100 μm. The 1000 μm diameter supporting base of our implant head could allow larger electrodes to be fitted if the current small sized electrodes were unable to provide long term stimulations below the retinal safety threshold.
In summary, we have implanted subretinal polyimide electrode platforms in the P23H rat, which provides an appropriate model for adRP in humans. Although the surgical technique is difficult because of the very small globe size, subretinal implants in the P23H rat should provide a valid model for assessing the electrical and biological modalities of subretinal electrode stimulation with the aim of developing a retinal prosthesis in humans.
Acknowledgements
The staff of the EPFL Center for MicroNanoTechnology is acknowledged for technical support in the fabrication of the electrodes.
Abbreviations
adRP - autosomal dominant retinitis pigmentosa
AMD - age related macular degeneration
MPDA - multiphotodiode array
RP - retinitis pigmentosa
RPE - retinal pigment epithelium
SLO - scanning laser ophthalmoscope
Footnotes
Funding: This work was supported by Swiss National Fund for Scientific Research (grants 3100–61956.00 and 3152–063915.00), Fondation en Faveur des Aveugles (Geneva), Fédération des aveugles et Handicapés Visuels de France, Fondation des Gueules Cassées, IRRP, Caisse d'Assurance Maladie des Professions Libérales, INSERM, University Pierre and Marie Curie (Paris VI), Assistance Publique‐Hôpitaux de Paris (AP‐HP), Fondation Ophtalmologique A de Rothschild.
Competing interests: none declared.
References
- 1.Stone J L, Barlow W E, Humayun M S.et al Morphometric analysis of macular photoreceptors and ganglion cells in retinas with retinitis pigmentosa. Arch Ophthalmol 19921101634–1639. [DOI] [PubMed] [Google Scholar]
- 2.Santos A, Humayun M S, de Juan M S.et al Preservation of the inner retina in retinitis pigmentosa. A morphometric analysis. Arch Ophthalmol 1997115511–515. [DOI] [PubMed] [Google Scholar]
- 3.Humayun M S, Prince M, de Juan E J.et al Morphometric analysis of the extramacular retina from post‐mortem eyes with with retinitis pigmentosa. Invest Ophthalmol Vis Sci 199640143–148. [PubMed] [Google Scholar]
- 4.Kim S Y, Sadda S, Pearlman J.et al Morphometric analysis of the macula in eyes with disciform age‐related macular degeneration. Retina 200222471–477. [DOI] [PubMed] [Google Scholar]
- 5.Kim S Y, Sadda S, Humayun M S.et al Morphometric analysis of the macula in eyes with geographic atrophy due to age‐related macular degeneration. Retina 200222464–470. [DOI] [PubMed] [Google Scholar]
- 6.Humayun M S, de Juan E, Jr, Dagnelie G.et al Visual perception elicited by electrical stimulation of retina in blind humans. Arch Ophthalmol 199611440–46. [DOI] [PubMed] [Google Scholar]
- 7.Humayun M S, de Juan E, Jr, Weiland J D.et al Pattern electrical stimulation of the human retina. Vis Res 1999392569–2576. [DOI] [PubMed] [Google Scholar]
- 8.Chow A Y, Chow V Y. Subretinal electrical stimulation of the rabbit retina.Neurosci Lett 199722513–16. [DOI] [PubMed] [Google Scholar]
- 9.Zrenner E, Miliczek K D, Gabel V P.et al The development of subretinal microphotodiodes for replacement of degenerated photoreceptors. Ophthalmic Res 199729269–280. [DOI] [PubMed] [Google Scholar]
- 10.Ziegler D, Linderholm P, Mazza M.et al An active microphotodiode array of oscillating pixels for retinal stimulation. Sens Actuators A Phys 200411011–17. [Google Scholar]
- 11.Kerdraon Y A, Downie J A, Suaning G J.et al Development and surgical implantation of a vision prosthesis model into the ovine eye. Clin Experiment Ophthalmol 20023036–40. [DOI] [PubMed] [Google Scholar]
- 12.Rizzo J F, 3rd, Wyatt J, Loewenstein J.et al ethods and perceptual thresholds for short‐term electrical stimulation of human retina with microelectrode arrays. Invest Ophthalmol Vis Sci 2003445355–5361. [DOI] [PubMed] [Google Scholar]
- 13.Rizzo J F, 3rd, Wyatt J, Loewenstein J.et al erceptual efficacy of electrical stimulation of human retina with a microelectrode array during short‐term surgical trials. Invest Ophthalmol Vis Sci 2003445362–5369. [DOI] [PubMed] [Google Scholar]
- 14.Eckmiller R. Learning retina implants with epiretinal contacts. Ophthalmic Res 199729281–289. [DOI] [PubMed] [Google Scholar]
- 15.Humayun M S. Intraocular retinal prosthesis. Trans Am Ophthalmol Soc 200199271–300. [PMC free article] [PubMed] [Google Scholar]
- 16.Humayun M S, Weiland J D, Fujii G Y.et al Visual perception in a blind subject with a chronic microelectronic retinal prosthesis. Vis Res 2003432573–2581. [DOI] [PubMed] [Google Scholar]
- 17.Veraart C, Raftopoulos C, Mortimer J T.et al Visual sensations produced by optic nerve stimulation using an implanted self‐sizing spiral cuff electrode. Brain Res 1998813181–186. [DOI] [PubMed] [Google Scholar]
- 18.Delbeke J, Oozeer M, Veraart C. Position, size and luminosity of phosphenes generated by direct optic nerve stimulation. Vis Res 2003431091–1102. [DOI] [PubMed] [Google Scholar]
- 19.Pardue M T, Stubbs E B, Jr, Perlman J.et al Immunohistochemical studies of the retina following long‐term implantation with subretinal microphotodiode arrays. Exp Eye Res 200173333–343. [DOI] [PubMed] [Google Scholar]
- 20.Pardue M T, Ball S L, Hetling J.et al Visual evoked potentials to infrared stimulation in normal cats and rats. Doc Ophthalmol 2001S103155–162. [DOI] [PubMed] [Google Scholar]
- 21.Schanze T, Sachs H G, Wiesenack C.et al Implantation and testing of subretinal film electrodes in domestic pigs. Exp Eye Res. 2005;[Epub ahead of print] [DOI] [PubMed]
- 22.Sachs H G, Schanze T, Wilms M. Subretinal implantation and testing of polyimide film electrodes in cats. Graefes Arch Clin Exp Ophthalmol 2005243464–468. [DOI] [PubMed] [Google Scholar]
- 23.Pardue M T, Phillips M J, Yin H.et al Neuroprotective effect of subretinal implants in the RCS rat. Invest Ophthalmol Vis Sci 200546674–682. [DOI] [PubMed] [Google Scholar]
- 24.Chow A Y, Chow V Y, Packo K H.et al The artificial silicon retina microchip for the treatment of vision loss from retinitis pigmentosa. Arch Ophthalmol 2004122460–469. [DOI] [PubMed] [Google Scholar]
- 25.Pardue M T, Phillips M J, Yin H.et al Neuroprotective effect of subretinal implants in the RCS rat. Invest Ophthalmol Vis Sci 200546674–682. [DOI] [PubMed] [Google Scholar]
- 26.Metz S, Bertsch A, Bertrand D.et al Flexible polyimide probes with microelectrodes and embedded microfluidic channels for simultaneous drug delivery and multi‐channel monitoring of bioelectric activity. Biosens Bioelectron 2004191309–1318. [DOI] [PubMed] [Google Scholar]
- 27.Banisadr G, Queraud‐Lesaux F, Boutterin M C.et al Distribution, cellular localization and functional role of CCR2 chemokine receptors in adult rat brain.J Neurochem 200281257–269. [DOI] [PubMed] [Google Scholar]
- 28.Schwahn H N, Gekeler F, Kohler K.et al Studies on the feasibility of a subretinal visual prosthesis: data from Yucatan micropig and rabbit. Graefes Arch Clin Exp Ophthalmol 2001239961–967. [DOI] [PubMed] [Google Scholar]
- 29.Chow A Y, Pardue M T, Chow V Y.et al Implantation of silicon chip microphotodiode arrays into the cat subretinal space. IEEE Trans Neural Syst Rehabil Eng 2001986–95. [DOI] [PubMed] [Google Scholar]
- 30.Hämmerle H, Kobuch K, Kohler K.et al Biostability of microphotodiode arrays for subretinal implantation. Biomaterials 200223797–804. [DOI] [PubMed] [Google Scholar]
- 31.Chow A Y, Pardue M T, Perlman J I.et al Subretinal implantation of semiconductor‐based photodiodes: durability of novel implant designs. J Rehabil Res Dev 200239312–322. [PubMed] [Google Scholar]
- 32.Zrenner E, Stett A, Weiss S.et al Can subretinal microphotodiodes successfully replace degenerated photoreceptors? Vis Res 1999392555–2567. [DOI] [PubMed] [Google Scholar]
- 33.Frasson M, Picaud S, Leveillard T.et al Glial cell line‐derived neurotrophic factor induces histologic and functional protection of rod photoreceptors in the rd/rd mouse. Invest Ophthalmol Vis Sci 1999402724–2734. [PubMed] [Google Scholar]
- 34.D'Cruz P M, Yasumura D.et al Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum Mol Genet 20009645–651. [DOI] [PubMed] [Google Scholar]
- 35.Machida S, Kondo M, Jamison J A.et al P23H rhodopsin transgenic rat: correlation of retinal function with histopathology. Invest Ophthalmol Vis Sci 2000413200–3209. [PubMed] [Google Scholar]
- 36.Zrenner E. The subretinal implant: can microphotodiode arrays replace degenerated retinal photoreceptors to restore vision? Ophthalmologica 2002216S1, 8–20 discussion 523. [DOI] [PubMed] [Google Scholar]
- 37.Baig‐Silva M S, Hathcock C D, Hetling J R. A preparation for studying electrical stimulation of the retina in vivo in rat. J Neural Eng 20052S29–38, Epub 2005 Feb 22. [DOI] [PubMed] [Google Scholar]
- 38.Grimnes S, Martinsen O.Bioimpedance and bioelectricity basics. London: Academic Press, 20003
- 39.Sommerhalder J, Oueghlani E, Bagnoud M.et al Simulation of artificial vision: I. Eccentric reading of isolated words, and perceptual learning. Vis Res 200343269–283. [DOI] [PubMed] [Google Scholar]
- 40.Sommerhalder J, Rappaz B, de Haller R.et al Simulation of artificial vision: II. Eccentric reading of full‐page text and the learning of this task. Vis Res 2004441693–1706. [DOI] [PubMed] [Google Scholar]