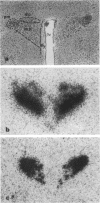
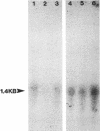
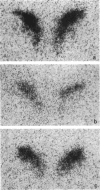
Abstract
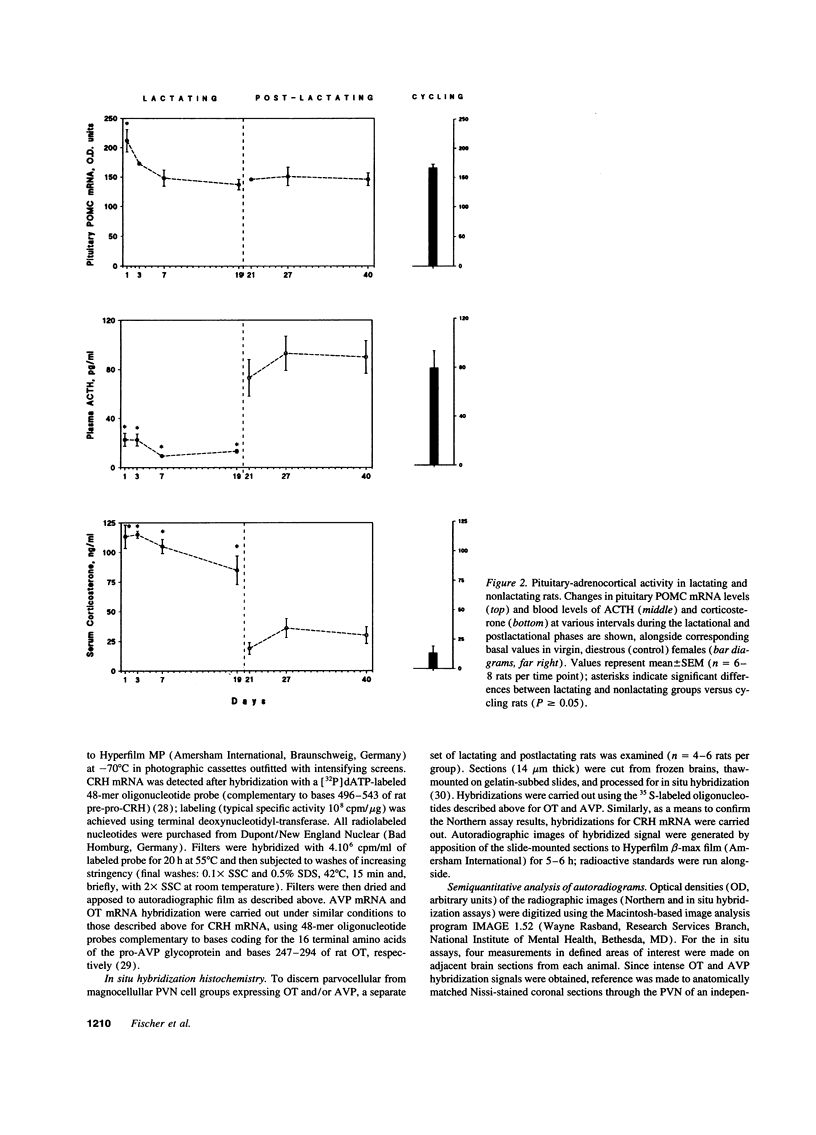
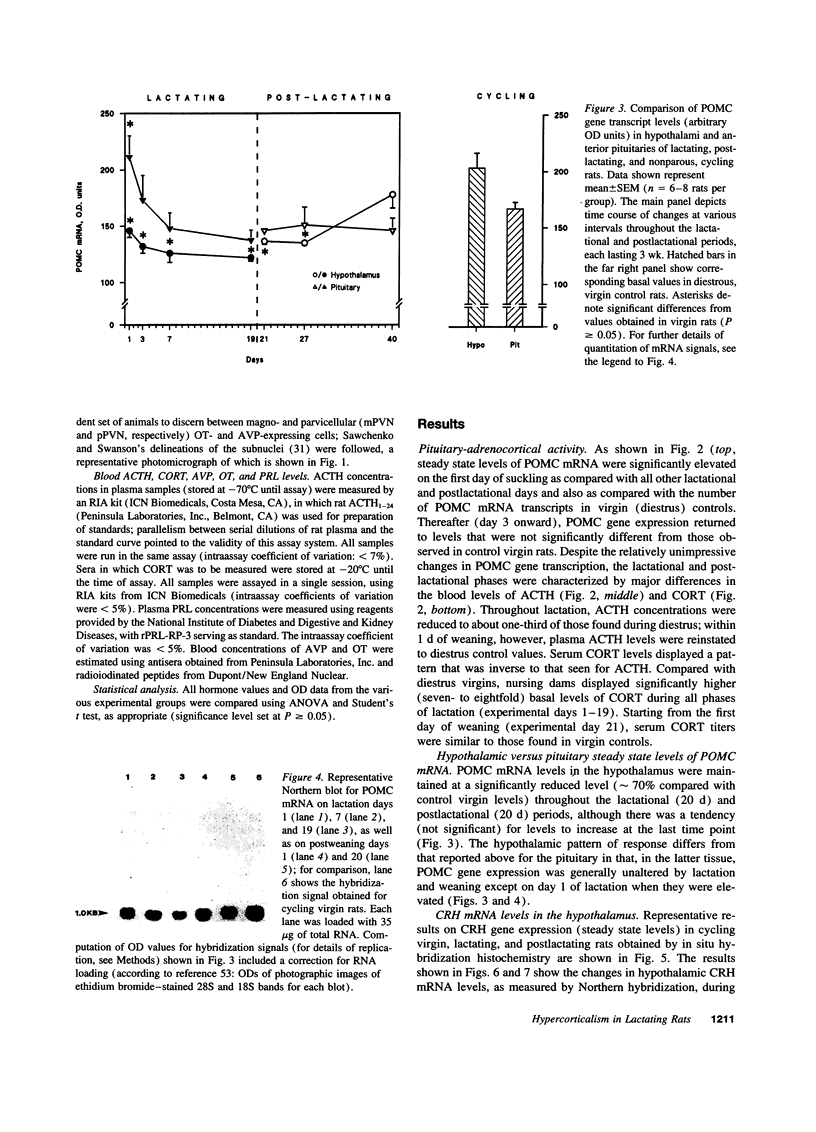
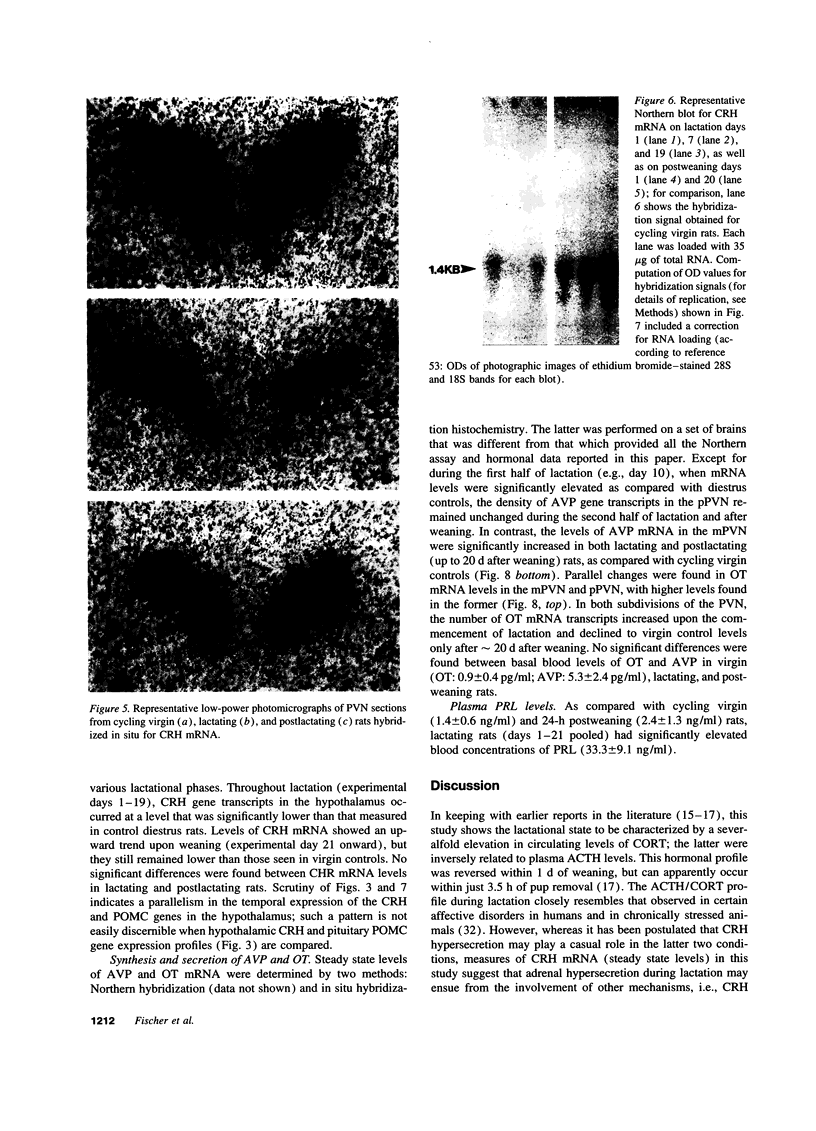
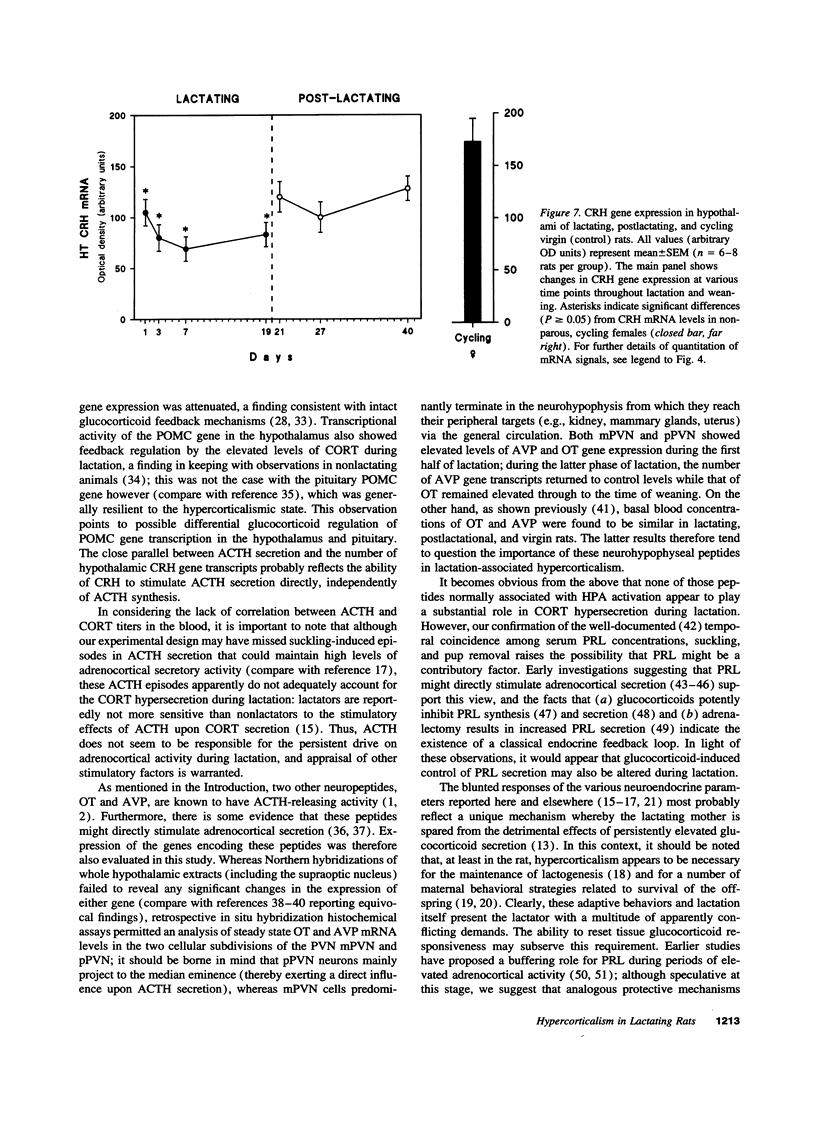
Steady state levels of hypothalamic expression of the genes encoding corticotropin-releasing hormone (CRH), proopiomelanocortin (POMC), arginine vasopressin (AVP), and oxytocin (OT) were studied in rats to investigate the mechanisms underlying the transitions between hypercorticalism during lactation and normocorticalism upon weaning. During lactation, CRH mRNA levels and blood titers of adrenocorticotropin (ACTH) were found to be significantly reduced, although POMC mRNA levels in the anterior pituitary were not significantly different from those found in cycling virgin (control) rats; during all phases of lactation, an inverse relationship was observed between the blood levels of ACTH and corticosterone (CORT). Plasma prolactin (PRL) concentrations were elevated approximately 30-fold during lactation. Whereas steady state levels of OT mRNA were markedly increased throughout lactation, those of AVP mRNA were only transiently (initially) elevated, and the blood levels of these hormones were not significantly altered in lactating as compared with cycling virgin and postlactating rats. CRH and POMC gene expression and blood levels of ACTH, CORT, and PRL were normalized within 1-3 d of removal of suckling pups. The temporal relationships between the biosynthetic profiles of the various peptide hormones and the patterns of ACTH and CORT secretion during the two physiological states suggest that lactation-associated hypercorticalism does not merely result from increased ACTH secretion; although still not well substantiated at this time, the evidence points to contributory roles of PRL, OT, and AVP in the hypercorticalismic state found during lactation.
Full text
PDF
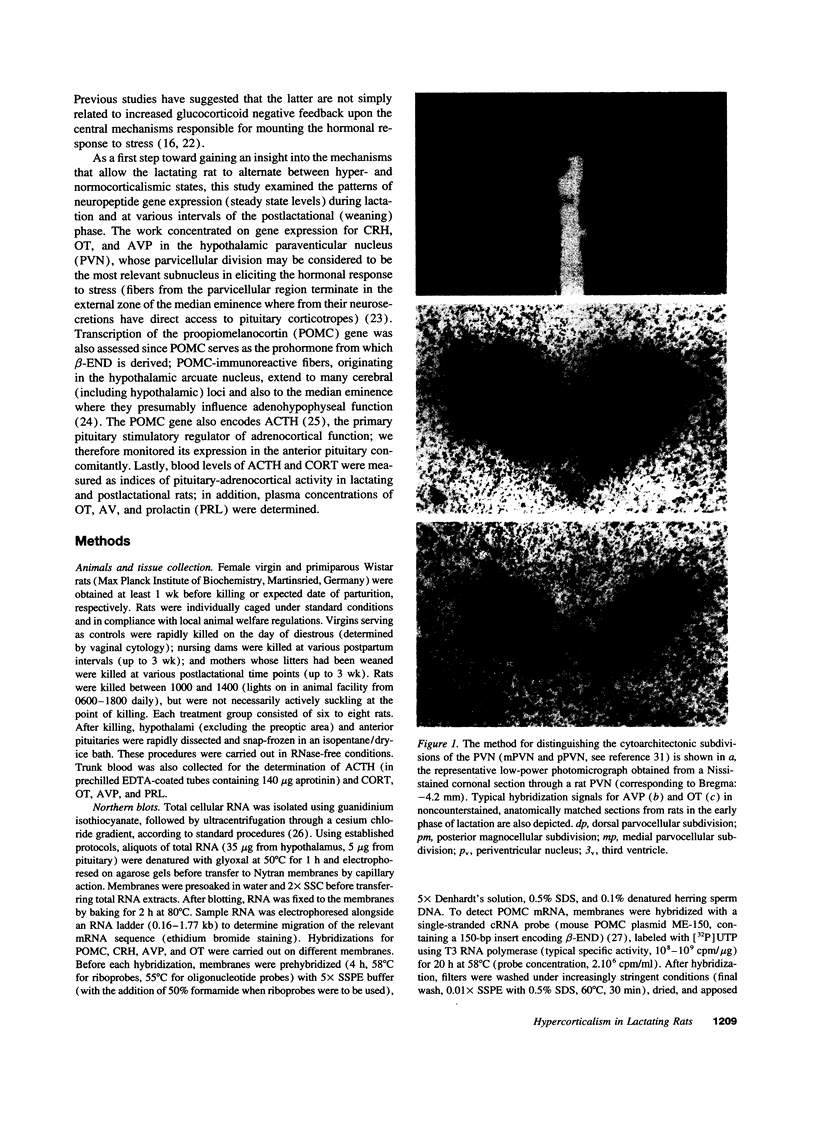

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almeida O. F., Hassan A. H., Holsboer F. Intrahypothalamic neuroendocrine actions of corticotropin-releasing factor. Ciba Found Symp. 1993;172:151–172. doi: 10.1002/9780470514368.ch8. [DOI] [PubMed] [Google Scholar]
- Antoni F. A., Palkovits M., Makara G. B., Linton E. A., Lowry P. J., Kiss J. Z. Immunoreactive corticotropin-releasing hormone in the hypothalamoinfundibular tract. Neuroendocrinology. 1983 Jun;36(6):415–423. doi: 10.1159/000123492. [DOI] [PubMed] [Google Scholar]
- Antoni F. A. Vasopressinergic control of pituitary adrenocorticotropin secretion comes of age. Front Neuroendocrinol. 1993 Apr;14(2):76–122. doi: 10.1006/frne.1993.1004. [DOI] [PubMed] [Google Scholar]
- Baldino F., Jr, Chesselet M. F., Lewis M. E. High-resolution in situ hybridization histochemistry. Methods Enzymol. 1989;168:761–777. doi: 10.1016/0076-6879(89)68057-3. [DOI] [PubMed] [Google Scholar]
- Bruhn T. O., Sutton S. W., Plotsky P. M., Vale W. W. Central administration of corticotropin-releasing factor modulates oxytocin secretion in the rat. Endocrinology. 1986 Oct;119(4):1558–1563. doi: 10.1210/endo-119-4-1558. [DOI] [PubMed] [Google Scholar]
- Correa-Rotter R., Mariash C. N., Rosenberg M. E. Loading and transfer control for Northern hybridization. Biotechniques. 1992 Feb;12(2):154–158. [PubMed] [Google Scholar]
- Crowley R. S., Insel T. R., O'Keefe J. A., Amico J. A. Cytoplasmic oxytocin and vasopressin gene transcripts decline postpartum in the hypothalamus of the lactating rat. Endocrinology. 1993 Dec;133(6):2704–2710. doi: 10.1210/endo.133.6.7612074. [DOI] [PubMed] [Google Scholar]
- Crowley W. R., Armstrong W. E. Neurochemical regulation of oxytocin secretion in lactation. Endocr Rev. 1992 Feb;13(1):33–65. doi: 10.1210/edrv-13-1-33. [DOI] [PubMed] [Google Scholar]
- Fitzsimmons M. D., Roberts M. M., Sherman T. G., Robinson A. G. Models of neurohypophyseal homeostasis. Am J Physiol. 1992 Jun;262(6 Pt 2):R1121–R1130. doi: 10.1152/ajpregu.1992.262.6.R1121. [DOI] [PubMed] [Google Scholar]
- Gillies G. E., Linton E. A., Lowry P. J. Corticotropin releasing activity of the new CRF is potentiated several times by vasopressin. Nature. 1982 Sep 23;299(5881):355–357. doi: 10.1038/299355a0. [DOI] [PubMed] [Google Scholar]
- Goebel S., Dietrich M., Jarry H., Wuttke W. Indirect evidence to suggest that prolactin mediates the adrenal action of haloperidol to stimulate aldosterone and corticosterone secretion in rats. Endocrinology. 1992 Feb;130(2):914–919. doi: 10.1210/endo.130.2.1310283. [DOI] [PubMed] [Google Scholar]
- Hansen S., Ferreira A. Food intake, aggression, and fear behavior in the mother rat: control by neural systems concerned with milk ejection and maternal behavior. Behav Neurosci. 1986 Feb;100(1):64–70. doi: 10.1037//0735-7044.100.1.64. [DOI] [PubMed] [Google Scholar]
- Hinson J. P., Vinson G. P., Porter I. D., Whitehouse B. J. Oxytocin and arginine vasopressin stimulate steroid secretion by the isolated perfused rat adrenal gland. Neuropeptides. 1987 Jul;10(1):1–7. doi: 10.1016/0143-4179(87)90083-7. [DOI] [PubMed] [Google Scholar]
- Holsboer F., Spengler D., Heuser I. The role of corticotropin-releasing hormone in the pathogenesis of Cushing's disease, anorexia nervosa, alcoholism, affective disorders and dementia. Prog Brain Res. 1992;93:385–417. doi: 10.1016/s0079-6123(08)64586-0. [DOI] [PubMed] [Google Scholar]
- Imaki T., Nahan J. L., Rivier C., Sawchenko P. E., Vale W. Differential regulation of corticotropin-releasing factor mRNA in rat brain regions by glucocorticoids and stress. J Neurosci. 1991 Mar;11(3):585–599. doi: 10.1523/JNEUROSCI.11-03-00585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivell R., Richter D. Structure and comparison of the oxytocin and vasopressin genes from rat. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2006–2010. doi: 10.1073/pnas.81.7.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson L., Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev. 1991 May;12(2):118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- Jingami H., Matsukura S., Numa S., Imura H. Effects of adrenalectomy and dexamethasone administration on the level of prepro-corticotropin-releasing factor messenger ribonucleic acid (mRNA) in the hypothalamus and adrenocorticotropin/beta-lipotropin precursor mRNA in the pituitary in rats. Endocrinology. 1985 Oct;117(4):1314–1320. doi: 10.1210/endo-117-4-1314. [DOI] [PubMed] [Google Scholar]
- Kiss J. Z., Mezey E., Skirboll L. Corticotropin-releasing factor-immunoreactive neurons of the paraventricular nucleus become vasopressin positive after adrenalectomy. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1854–1858. doi: 10.1073/pnas.81.6.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaer A. Vasopressin as a neuroendocrine regulator of anterior pituitary hormone secretion. Acta Endocrinol (Copenh) 1993 Dec;129(6):489–496. doi: 10.1530/acta.0.1290489. [DOI] [PubMed] [Google Scholar]
- Lau C. Effects of various stressors on milk release in the rat. Physiol Behav. 1992 Jun;51(6):1157–1163. doi: 10.1016/0031-9384(92)90302-i. [DOI] [PubMed] [Google Scholar]
- Lightman S. L. Alterations in hypothalamic-pituitary responsiveness during lactation. Ann N Y Acad Sci. 1992 Jun 12;652:340–346. doi: 10.1111/j.1749-6632.1992.tb34365.x. [DOI] [PubMed] [Google Scholar]
- Lightman S. L., Young W. S., 3rd Lactation inhibits stress-mediated secretion of corticosterone and oxytocin and hypothalamic accumulation of corticotropin-releasing factor and enkephalin messenger ribonucleic acids. Endocrinology. 1989 May;124(5):2358–2364. doi: 10.1210/endo-124-5-2358. [DOI] [PubMed] [Google Scholar]
- Maestripieri D., Badiani A., Puglisi-Allegra S. Prepartal chronic stress increases anxiety and decreases aggression in lactating female mice. Behav Neurosci. 1991 Oct;105(5):663–668. doi: 10.1037//0735-7044.105.5.663. [DOI] [PubMed] [Google Scholar]
- Mann P. E., Bridges R. S. Neural and endocrine sensitivities to opioids decline as a function of multiparity in the rat. Brain Res. 1992 May 15;580(1-2):241–248. doi: 10.1016/0006-8993(92)90950-e. [DOI] [PubMed] [Google Scholar]
- Marubayashi U., McCann S. M., Antunes-Rodrigues J. Factors controlling adrenal weight and corticosterone secretion in male rats as revealed by median eminence lesions and pharmacological alteration of prolactin secretion. Brain Res Bull. 1987 Nov;19(5):511–518. doi: 10.1016/0361-9230(87)90066-9. [DOI] [PubMed] [Google Scholar]
- Muir J. L., Pfister H. P. Influence of exogenously administered oxytocin on the corticosterone and prolactin response to psychological stress. Pharmacol Biochem Behav. 1988 Apr;29(4):699–703. doi: 10.1016/0091-3057(88)90190-6. [DOI] [PubMed] [Google Scholar]
- Neumann I., Russell J. A., Landgraf R. Oxytocin and vasopressin release within the supraoptic and paraventricular nuclei of pregnant, parturient and lactating rats: a microdialysis study. Neuroscience. 1993 Mar;53(1):65–75. doi: 10.1016/0306-4522(93)90285-n. [DOI] [PubMed] [Google Scholar]
- Owens M. J., Nemeroff C. B. The role of corticotropin-releasing factor in the pathophysiology of affective and anxiety disorders: laboratory and clinical studies. Ciba Found Symp. 1993;172:296–316. doi: 10.1002/9780470514368.ch15. [DOI] [PubMed] [Google Scholar]
- Patel H., Chowdrey H. S., Lightman S. L. Lactation abolishes corticotropin-releasing factor-induced oxytocin secretion in the conscious rat. Endocrinology. 1991 Feb;128(2):725–727. doi: 10.1210/endo-128-2-725. [DOI] [PubMed] [Google Scholar]
- Sawchenko P. E., Swanson L. W. The organization of forebrain afferents to the paraventricular and supraoptic nuclei of the rat. J Comp Neurol. 1983 Aug 1;218(2):121–144. doi: 10.1002/cne.902180202. [DOI] [PubMed] [Google Scholar]
- Schlein P. A., Zarrow M. X., Denenberg V. H. The role of prolactin in the depressed or 'buffered' adrenocorticosteroid response of the rat. J Endocrinol. 1974 Jul;62(1):93–99. doi: 10.1677/joe.0.0620093. [DOI] [PubMed] [Google Scholar]
- Schlosser S. F., Almeida O. F., Patchev V. K., Yassouridis A., Elands J. Oxytocin-stimulated release of adrenocorticotropin from the rat pituitary is mediated by arginine vasopressin receptors of the V1b type. Endocrinology. 1994 Nov;135(5):2058–2063. doi: 10.1210/endo.135.5.7956927. [DOI] [PubMed] [Google Scholar]
- Somasekhar M. B., Gorski J. Two elements of the rat prolactin 5' flanking region are required for its regulation by estrogen and glucocorticoids. Gene. 1988 Sep 15;69(1):13–21. doi: 10.1016/0378-1119(88)90373-3. [DOI] [PubMed] [Google Scholar]
- Stalla G. K., Stalla J., Huber M., Loeffler J. P., Höllt V., von Werder K., Müller O. A. Ketoconazole inhibits corticotropic cell function in vitro. Endocrinology. 1988 Feb;122(2):618–623. doi: 10.1210/endo-122-2-618. [DOI] [PubMed] [Google Scholar]
- Stern J. M., Goldman L., Levine S. Pituitary-adrenal responsiveness during lactation in rats. Neuroendocrinology. 1973;12(3):179–191. doi: 10.1159/000122167. [DOI] [PubMed] [Google Scholar]
- Stern J. M., Levine S. Psychobiological aspects of lactation in rats. Prog Brain Res. 1974;41:433–444. doi: 10.1016/S0079-6123(08)61924-X. [DOI] [PubMed] [Google Scholar]
- Svare B., Bartke A., Doherty P., Mason I., Michael S. D., Smith M. S. Hyperprolactinemia suppresses copulatory behavior in male rats and mice. Biol Reprod. 1979 Sep;21(2):529–535. doi: 10.1095/biolreprod21.2.529. [DOI] [PubMed] [Google Scholar]
- Walker C. D., Lightman S. L., Steele M. K., Dallman M. F. Suckling is a persistent stimulus to the adrenocortical system of the rat. Endocrinology. 1992 Jan;130(1):115–125. doi: 10.1210/endo.130.1.1309321. [DOI] [PubMed] [Google Scholar]
- Watanobe H. The immunostaining for the hypothalamic vasoactive intestinal peptide, but not for beta-endorphin, dynorphin-A or methionine-enkephalin, is affected by the glucocorticoid milieu in the rat: correlation with the prolactin secretion. Regul Pept. 1990 May 21;28(3):301–311. doi: 10.1016/0167-0115(90)90028-u. [DOI] [PubMed] [Google Scholar]
- Wolfson B., Manning R. W., Davis L. G., Arentzen R., Baldino F., Jr Co-localization of corticotropin releasing factor and vasopressin mRNA in neurones after adrenalectomy. Nature. 1985 May 2;315(6014):59–61. doi: 10.1038/315059a0. [DOI] [PubMed] [Google Scholar]