Abstract
Aims
To evaluate rates of vitreous relapse among retinoblastoma patients treated with primary chemotherapy and assess diode laser as a potential risk factor for relapse.
Methods
Retrospective review of all patients treated with primary chemotherapy at a large ocular oncology centre. Eyes that developed vitreous relapse were coded with regard to Reese‐Ellsworth Group, laterality, time to relapse, type of relapse (vitreous base or non‐vitreous base relapse), treatments used (including adjuvant diode laser), and ocular preservation. Individual tumour foci treated with laser hyperthermia were also coded for laser parameters including power settings, number of treatments, and concomitant administration of systemic chemotherapy (chemothermotherapy).
Results
15 of 106 eyes (14.15%) developed vitreous relapse over a 6 year period. Mean time to relapse was 7.2 months after chemotherapy was completed. Five cases (33%) were of the vitreous base variety. Ocular salvage was attempted in 11 cases using a variety of methods; one patient was lost to follow up. Six of the remaining 10 eyes (60%) were salvaged. Eight of 38 eyes (21%) treated with systemic chemotherapy and laser hyperthermia developed vitreous relapse compared with seven of 68 eyes (10%) treated with primary chemotherapy alone (p<0.005). Laser settings, number of hyperthermia treatments, and the concomitant use of systemic chemotherapy (chemothermotherapy) were not associated with higher rates of vitreous relapse.
Conclusion
Nearly one in seven eyes with retinoblastoma treated with primary chemotherapy may develop vitreous relapse. The administration of diode laser hyperthermia appears to increase this risk. Despite additional therapy a number of these eyes succumb to enucleation.
Keywords: retinoblastoma, chemotherapy, vitreous relapse, laser hyperthermia, transpupillary thermotherapy, children
Since the mid‐1990s chemotherapy has become the primary eye sparing modality used to treat intraocular retinoblastoma. Its effectiveness has been demonstrated by numerous studies among various Reese‐Ellsworth groups. Many patients are cured with this approach and thereby avoid the related morbidity associated with external beam radiotherapy.1,2,3,4,5,6
Most centres administer a triple agent regimen consisting of carboplatin, vincristine, and etoposide combined with adjuvant treatment including diode laser hyperthermia, cryotherapy, and radiotherapy.7,8,9,10,11,12,13 However, despite being highly effective tumour foci seeding the vitreous can be resistant to many of these methods.14,15,16 Our service and others have examined the response rates of eyes presenting with vitreous disease (Reese‐Ellsworth Group Vb)2,4,5; some centres have reported the use of periocular chemotherapy in this setting.17 Few studies however have investigated vitreous relapse—the failure of first line therapy with secondary seeding of the vitreous—following primary chemotherapy.
The purpose of this report was to assess the incidence of vitreous relapse among patients treated with primary chemotherapy at a large ocular oncology centre and evaluate their response to various adjuvant methods. Having identified this cohort of patients we then sought to assess which risk factors are associated with vitreous relapse.
Methods
A retrospective review was performed using the records of the Ocular Oncology Service of St Bartholomew's Hospital, London. Included were all children diagnosed with intraocular retinoblastoma and treated with primary chemotherapy from June 1994 until June 2000. Our regimen included carboplatin, vincristine, and etoposide given at 21 day intervals and has been published elsewhere.4 Hyperthermia (when indicated) was administered in standard fashion using an 810 nm diode laser coupled to an operating microscope and contact lens.8 The decision to use this modality was made on a case by case basis by one of the authors (JLH) for tumours with residual activity following chemoreduction. Eyes presenting with vitreous disease (Reese Group Vb) and those cases treated by other methods (enucleation or primary external beam radiotherapy) were excluded from this study.
Eyes were coded with regard to laterality, age at diagnosis, Reese‐Ellsworth group, treatment with laser hyperthermia, and follow up time. The presence of vitreous relapse was assessed by one of the authors (JLH). These cases were coded for type of relapse (vitreous base or non‐vitreous base recurrence), time to relapse (from initial administration of chemotherapy and from completion of chemotherapy), treatment used, and ocular preservation. Individual tumour foci treated with laser hyperthermia were also coded for size, laser setting, and the concomitant administration of systemic chemotherapy (chemothermotherapy). Tumour size was the largest basal diameter in millimetres as estimated by one of the authors (JLH) using indirect ophthalmoscopy and a 20 dioptre lens. Laser parameters included spot size (in mm), energy settings (in mW), duration, and number of treatments.
A multivariate analysis was performed using a series of Cox proportional hazard regressions. The analysis was carried out using the statistical package Stata version 6.0.
Results
Vitreous relapse and rates of ocular salvage
In all, 106 eyes in 84 patients met the inclusion criteria of this study. Age at enrolment ranged from 1–62 months (mean 23.8 months, median 9 months). Fifteen eyes (14.15%) developed vitreous relapse (as confirmed by one of the authors, JLH). Five (33%) were of the vitreous base type. Thirteen cases occurred in patients with bilateral disease. Five eyes (33%) presented with Reese‐Ellsworth Group 2a disease. Three eyes were stage 3a, two were 2b and 4b, and the remaining were stages 1a, 3b, and 5a. Average time to relapse was 13.7 months following the initial administration of chemotherapy (range 4–37 months, median 11 months) and 7.2 months following completion of chemotherapy (range 0–29 months, median 4 months). Three eyes (20%) relapsed while receiving chemotherapy (see table 1).
Table 1 Cohort of eyes with vitreous relapse following primary chemotherapy.
| Case no | Eye | R‐E group* | Chemo† | Time to relapse following completion of chemotherapy (months) | Treatment used | Eye salvaged | |
|---|---|---|---|---|---|---|---|
| 1 | R | 3b | No | 3 | Iridium wire | Lost to follow up | |
| 2 | R | 2b | No | 1 | EBRT‡ | Yes | |
| 3 | R | 2a | No | 4 | Cryotherapy | Yes | |
| 4 | R | 2a | No | 6 | EBRT, cryotherapy | Yes | |
| 5 | R | 5a | No | 10 | EBRT | Yes | |
| 6 | R | 1a | No | 29 | Periocular chemotherapy | No | |
| 7 | R | 3a | No | 4 | Iridium wire | Yes | |
| 8 | R | 3a | No | 18 | EBRT, iridium wire | No | |
| 9 | R | 2a | No | 22 | Periocular chemotherapy | No | |
| 10 | R | 3a | Yes | 0 | EBRT, periocular chemotherapy | No | |
| 11 | R | 2b | Yes | 0 | Completion of systemic chemotherapy | Yes | |
| 12 | R | 4b | No | 1 | Enucleation | NA | |
| 13 | R | 4b | No | 1 | Enucleation | NA | |
| 14 | R | 2a | No | 9 | Enucleation | NA | |
| 15 | R | 2a | Yes | 0 | Enucleation | NA |
*Reese‐Ellsworth group at presentation.
†Was patient receiving systemic chemotherapy when relapse occurred?
‡External beam radiotherapy.
Four eyes were treated by enucleation. (Two patients had unilateral disease, the other two were bilateral patients where the contralateral eye was in remission with good visual potential.) Ocular salvage was attempted in the remaining cases. The modality used was selected by our senior ophthalmologist on an individual basis. One patient (case 1) was treated with iridium wire and was lost to follow up. Of the remaining 10 eyes four succumbed to enucleation (40%). Follow up time for the remaining eyes (after salvage) ranged from 6–51 months with a mean of 26 months (median 19 months).
Risk of vitreous relapse associated with laser administration
A total of 47 tumours in 38 eyes received diode laser hyperthermia in addition to chemotherapy. They ranged in basal diameter from 2–31 mm (mean 8 mm, median 5.7 mm). There was a total of 87 laser sessions, of which 69 were concomitantly administered with systemic chemotherapy (chemothermotherapy). Individual tumours averaged 1.85 laser treatments (range 1–4 sessions, median 2). Mean laser settings were 578 mW (range 300–860 mW, median 600 mW) for 9 minutes (range 22–1200 seconds, median 321 seconds) with a spot size of 1.2 mm (range 0.3–2.0 mm, median 1.2 mm).
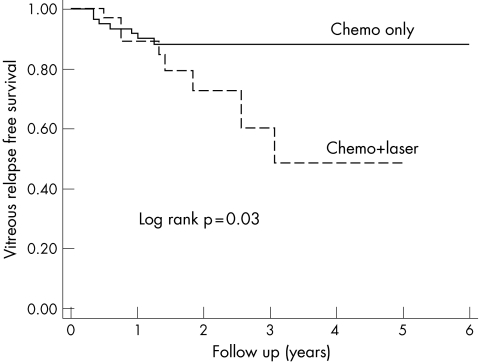
Eight of 38 eyes (21%) treated with systemic chemotherapy and laser hyperthermia developed vitreous relapse compared with seven of 68 eyes (10%) treated with primary chemotherapy alone (p<0.005, 95% confidence interval: 1.57 to 12.3). Those eyes treated with laser hyperthermia relapsed 1–20 months after initial laser application (mean 2.9 months, median 7 months). Table 2 lists the eyes per Reese‐Ellsworth group and treatment with or without laser hyperthermia. The probability of remaining free of vitreous relapse was estimated by Kaplan‐Meier survival curves (fig 2).
Table 2 Initial Reese‐Ellsworth group of eyes developing vitreous relapse treated with and without diode laser.
| Reese‐Ellsworth group | Total no of eyes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ia | Ib | IIa | IIb | IIIa | IIIb | IVa | IVb | Va | Vb | ||
| Eyes treated with diode laser | 0 | 1 | 1 | 1 | 3 | 1 | 0 | 0 | 1 | 0 | 8 |
| Eyes treated without diode laser | 0 | 0 | 4 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 7 |
Figure 2 Kaplan‐Meier survival curves for vitreous relapse in eyes treated with chemotherapy alone and eyes treated with chemotherapy and diode laser.
Laser settings, number of hyperthermia treatments, and the concomitant use of systemic chemotherapy (chemothermotherapy) were not associated with higher rates of vitreous relapse. Laterality (left eye) was found to correlate with development of vitreous relapse on univariate and multivariate analysis (p<0.095, 95% confidence interval 0.12 to 1.18).
Discussion
Over the past decade, chemotherapy has replaced radiotherapy as the preferred eye preserving primary method for retinoblastoma. This approach avoids the known toxicity of external beam radiotherapy with similar rates of ocular retention. However, curing retinoblastoma with vitreous involvement has always been challenging. In this study we reviewed our experience in eyes failing primary chemotherapy with vitreous relapse. Our aims were to establish incidence rates and methods effective in salvaging these eyes. In addition, we identified one of many potential risk factors related to this phenomenon—application of diode laser hyperthermia.
The management of retinoblastoma with primary chemotherapy is highly individualised. Many factors are considered in choosing which methods are used in any individual patient. In order to avoid selection bias we reviewed every patient on our service treated with primary chemotherapy irrespective of which adjuvant therapy (if any) was used. We excluded eyes with vitreous disease on presentation and those that were treated by other primary methods.
We analysed multiple variables including Reese‐Ellsworth group, tumour size, laser settings (power, spot size, duration of treatment), number of treatment applications, and concomitant use of laser therapy and systemic chemotherapy (chemothermotherapy) in an attempt to isolate which factors pose a higher risk of relapse. Our findings demonstrated a significant rate of vitreous relapse in our cohort and the application of diode laser as a risk factor associated with this entity.
Vitreous relapse
We found that nearly one in seven eyes treated with primary chemotherapy eventually developed vitreous relapse, an incidence of 14.15% (see fig 1). Such a high rate was surprising but not entirely unexpected as we have noted an increase in this phenomenon since the decline of primary external beam radiotherapy (EBRT). Reports of vitreous recurrence in eyes without Vb disease were rare following EBRT.18,19,20,21 In our cohort the mean time to relapse was 7.2 months following completion of chemotherapy but occurred as long as 2 years later. This demonstrates the potential for vitreous relapse to occur long after patients have initially responded to primary chemotherapy and illustrates the need for close serial follow up. Relapse occurred among all Reese‐Ellsworth groups; eyes with advanced stage were not at higher risk for this complication. Over half the cases (8/15) occurred in groups 1a, 2a, and 2b eyes that might have had a better prognosis had they been treated with primary EBRT. We acknowledge that some of the salvaged eyes had a shorter follow up time and would benefit from further observation to ensure that they remain in remission.
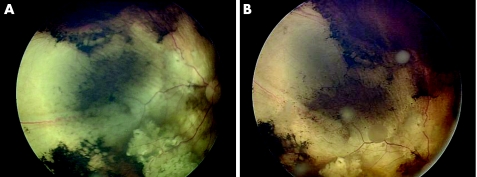
Figure 1 Fundus photographs of an eye with vitreous relapse following administration of systemic chemotherapy. (A) Demonstrates initial regression and (B) demonstrates diffuse vitreous relapse months later.
Four of the eyes in this study were treated with enucleation and further therapy was not recommended. We believe that this approach is reasonable for most unilateral retinoblastoma patients where surgery provides a high cure rate and limited morbidity. Among bilateral patients enucleation is also a sensible option in cases where the contralateral eye is in remission with good visual potential. We prefer to avoid EBRT in patients who harbour the retinoblastoma gene given their propensity for second non‐ocular tumours.6
Treatment of vitreous relapse
Salvage was attempted in 11 eyes. All of these patients had bilateral retinoblastoma; their contralateral eye was either enucleated or had a poor visual prognosis. Treatment options in these cases include brachytherapy, external beam radiotherapy, cryotherapy, additional systemic or periocular chemotherapy. Most of our patients received a combination of salvage therapies illustrating the difficulty of achieving tumour remission with a single method (see table 1). Distinguishing cases where relapse was limited to the vitreous base allowed us to select eyes amenable to brachytherapy with iridium wire.16 Five relapses (one third) were of the vitreous base type and three patients were treated with this approach. The majority of patients (7/11) were treated with some form of radiotherapy. Among the six eyes salvaged three were treated with EBRT and one with iridium wire. None of the three eyes injected with periocular chemotherapy were salvaged. While our cohort of treated patients is small the data suggest that radiotherapy can be used effectively following vitreous relapse. We caution, however, that further follow up is necessary to assess whether these eyes remain in remission.
Among the 11 patients treated for vitreous relapse one returned to his country of origin and was lost to follow up. Of the remaining eyes, four were enucleated resulting in a salvage rate of 60%. The high failure rate illustrates current limitations in curing this type of recurrence.
Risk factors for vitreous relapse
Notwithstanding the need for more effective therapy, establishing the risk factors associated with vitreous relapse is equally important. Factors associated with vitreous relapse may include the size, location, and inherent biology of individual retinoblastoma foci. Current chemotherapeutic regimens may select for more aggressive tumours (chemoresistance) with a predisposition for vitreous seeding. Some centres administer ciclosporin A in an attempt to reduce chemoresistance. Alternatively adjuvant therapy such as diode laser hyperthermia may pose an independent risk.
Case reports of vitreous relapse were described following xenon arc photocoagulation. High energy applications were thought to disperse malignant cells into the vitreous body following rupture of the internal limiting membrane (ILM). Diode laser is thought to cause cellular destruction at subphotocoagulation levels.22 Yet despite treatment at lower temperatures, laser hyperthermia may cause tumour disruption with similar effects as photocoagulation.
Alternatively, tumour biology and the initial response to chemotherapy may have a role. In our service the majority of tumours are treated first with systemic chemotherapy. Those with an inadequate response are administered an adjuvant method such as laser hyperthermia. It may be that this approach selects for tumour foci that are biologically prone to vitreous seeding. Cells that remain active following triple agent chemotherapy may spread with greater ease into the vitreous cavity following low dose administration of diode laser. In our study, eyes treated with diode laser were not randomised, all had initially received primary chemotherapy. Therefore, we acknowledge a selection bias potentially contributing to our results.
Clinical implications
These findings have significant clinical implications. Laser hyperthermia is used at the majority of ocular oncology centres in both primary and adjuvant settings. Other services have reported their experience with primary chemotherapy yet few have suggested similar rates of vitreous relapse, even with adjuvant laser.2,7,8,9,10,11,12,13,14 We suspect that differences in our patient population, laser administration and follow up time may be a factor in these findings.
We were among a handful of oncology centres that converted to primary chemotherapy in the early 1990s. With some relapses detected years after initial therapy it has taken nearly a decade to demonstrate these results. We suspect that as smaller oncology services gain additional experience their rates of vitreous relapse may increase in a similar fashion.
Our patient population in the United Kingdom may also be significant. Diode laser has greater absorption in pigmented tissue. Those patients who are fair skinned often require laser applications at higher energy levels for longer duration. These make up the majority of patients in our service. While we did not detect a specific energy setting that correlated with relapse it may be that overall, our patients require higher energy settings for longer periods than other services with a more heterogeneous population. Higher energy settings may be an additional factor associated with microscopic dispersion of cells into the vitreous.
Initial studies of chemothermotherapy described the application of laser hyperthermia with a microscope adaptor.8 This approach allows for application of spot sizes of 0.3–2.0 mm and is the preferred method on our service. Modification of this technique varies considerably among oncology centres. Some prefer the indirect ophthalmoscope attachment, others a transscleral probe.7,8,9,10,11,12,13,14 These approaches have a smaller and fixed spot size. Laser treatment with the microscope adaptor is best suited for posterior pole (versus peripheral) lesions. Thus the majority of tumours treated in this study were posterior to the equator. While we did not find spot size a significant risk factor for vitreous relapse overall, our mean spot size (1.2 mm) remained larger than those obtained with other laser attachments. These differences in laser technique (microscope attachment, tumour location, spot size) may affect the distribution and total energy delivered to tumour foci. It makes direct comparison between our data and other reported studies challenging.
As a result of our findings we have become more cautious in our use of adjuvant diode laser hyperthermia. If possible we avoid routine laser for those tumours that have responded well to primary chemotherapy, particularly in the macula. We use the lowest energy settings for the shortest time interval until we observe adequate tumour response. Whenever possible we use alternative forms of adjuvant therapy, including brachytherapy and cryotherapy.
Although new strategies for retinoblastoma have brought with them great benefit, their long term implications are still uncertain. Vitreous relapse after primary chemotherapy is a serious complication that can be difficult to control. Our study highlights certain areas of concern, which we shall continue to monitor closely.
Acknowledgements
We gratefully acknowledge the clerical assistance of Ms P Meelapsom and Ms P Jackson. This research was funded in part by a grant from The Retinoblastoma Society UK (DSG).
Abbreviations
EBRT - external beam radiotherapy
ILM - internal limiting membrane
TTT - transpupillary thermotherapy
References
- 1.Shields C L, Shields J A. Recent developments in the management of retinoblastoma. J Pediatr Ophthalmology Strabismus 1999368–18. [DOI] [PubMed] [Google Scholar]
- 2.Gallie B L, Budning A, DeBoer G.et al Chemotherapy with focal therapy can cure intraocular retinoblastoma without radiation. Arch Ophthalmology 19961141321–1329. [DOI] [PubMed] [Google Scholar]
- 3.Beck M N, Balmer A, Dessing C.et al First‐line chemotherapy with local treatment can prevent external‐beam irradiation and enucleation in low‐stage intraocular retinoblastoma. J Clin Oncol 2000182881–2887. [DOI] [PubMed] [Google Scholar]
- 4.Kingston J E, Hungerford J L, Madreperla S A.et al Results of combined chemotherapy and radiotherapy for advanced intraocular retinoblastoma. Arch Ophthalmol 19961141339–1343. [DOI] [PubMed] [Google Scholar]
- 5.Gunduz K, Shields C L, Shields J A.et al The outcome of chemoreduction treatment in patients with Reese‐Ellsworth group V retinoblastoma. Arch Ophthalmol 19981161613–1617. [DOI] [PubMed] [Google Scholar]
- 6.Abramson D H, Frank C M. Second nonocular tumours in survivors of bilateral retinoblastoma; a possible age effect on radiation‐related risk. Ophthalmology 1998105573–579. [DOI] [PubMed] [Google Scholar]
- 7.Levy C, Doz F, Quintana E.et al Role of chemotherapy alone or in combination with hyperthermia in the primary treatment of intraocular retinoblastoma: preliminary results. Br J Ophthalmol 1998821154–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphree A L, Villablanca J G.et al Chemotherapy plus local treatment in the management of intraocular retinoblastoma. Arch Ophthalmol 19961141348–1356. [DOI] [PubMed] [Google Scholar]
- 9.Shields C L, Shields J A, Needle M.et al Combined chemoreduction and adjuvant treatment for intraocular retinoblastoma. Ophthalmology 19971042101–2111. [DOI] [PubMed] [Google Scholar]
- 10.Brichard B, De Bruycker J J, De Potter P.et al Combined chemotherapy and local treatment in the management of intraocular retinoblastoma. Med Pediatr Oncol 200238411–415. [DOI] [PubMed] [Google Scholar]
- 11.Shields C L, Santos M C, Diniz W.et al Thermotherapy for retinoblastoma. Arch Ophthalmol 1999117885–893. [DOI] [PubMed] [Google Scholar]
- 12.Schueler A O, Jurklies C, Heimann H.et al Thermochemotherapy in hereditary retinoblastoma. Br J Ophthalmol 20038790–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lumbroso L, Doz F, Urbieta M.et al Chemothermotherapy in the management of retinoblastoma. Ophthalmology 20021091130–1136. [DOI] [PubMed] [Google Scholar]
- 14.Shields C L, Honavar S G, Meadows A T.et al Chemoreduction plus focal therapy for retinoblastoma: factors predictive of need for treatment with external beam radiotherapy or enucleation. Am J Ophthalmol 2002133657–664. [DOI] [PubMed] [Google Scholar]
- 15.Shields C L, Honavar S G, Shields J A.et al Factors predictive of recurrence of retinal tumors, vitreous seeds, and subretinal seeds following chemoreduction for retinoblastoma. Arch Ophthalmol 2002120460–464. [PubMed] [Google Scholar]
- 16.Madreperla S A, Hungerford J L, Doughty D.et al Treatment of retinoblastoma vitreous base seeding. Ophthalmology 1998105120–124. [DOI] [PubMed] [Google Scholar]
- 17.Abramson D H, Frank C M, Dunkel I J. A phase I/II study of subconjunctival carboplatin for intraocular retinoblastoma. Ophthalmology 19991061947–1950. [DOI] [PubMed] [Google Scholar]
- 18.Abramson D H, Greenfield D S, Ellsworth R M. Bilateral retinoblastoma. Correlations between age at diagnosis and time course for new intraocular tumors. Ophthal Pediatr Genet 1991131–7. [DOI] [PubMed] [Google Scholar]
- 19.Bedford M A, Bedotto D, Mac Faul P D. Retinoblastoma: a study of 139 cases. Br J Ophthalmol 19715519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salmonsen P C, Ellsworth R M, Ktichen F D. The occurrence of new retinoblastoma after treatment. Trans Am Acad Ophthalmol Otolaryngol 197986837–840. [Google Scholar]
- 21.Hernandez J C, Brady L W, Shields J A.et al External beam radiation for retinoblastoma: Results, patterns of failure and a proposal for treatment guidelines. Int J Radiation Oncol Biol Phys 199635125–132. [DOI] [PubMed] [Google Scholar]
- 22.Inomata M, Kaneko A, Kunimoto T.et al In vitro thermo‐ and thermochemo‐sensitivity of retinoblastoma cells from surgical specimens. Int J Hyperther 20021850–61. [DOI] [PubMed] [Google Scholar]