Pterygia represent growth onto the cornea of fibrovascular tissue continuous with the conjunctiva.1 KL‐6 (Krebs von den Lunge‐6) is a high molecular weight mucinous glycoprotein, and the monoclonal antibody reacts with the sugar moiety of MUC‐1.2,3 We have reported that measurement of serum KL‐6 levels is useful for the diagnosis and management of uveitis patients with sarcoidosis.4,5 The aim of this study was to examine the expression of KL‐6, and Ki‐67, a proliferation marker, in normal human conjunctiva, pterygium, and pseudopterygium tissues.
Methods
Five samples consisting of one normal conjunctiva, three pterygia, and one pseudopterygium were surgically collected. Formalin fixed and paraffin embedded tissue sections were incubated with anti‐KL‐6 and anti‐Ki‐67 monoclonal antibodies, and then examined immunohistochemically.
Results
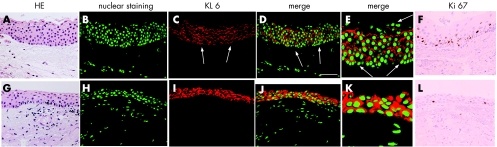
Immunoreactivity for KL‐6 was detected on the apical membrane of the wing and basal cells in the normal conjunctiva. In the human pterygium head, immunoreactivity for KL‐6 was observed on the apical membrane and in cytoplasm of the epithelium (fig 1A–E). In the pterygium body, immunoreactivity for KL‐6 was strongly detected in cytoplasm and/or circumferential membrane of epithelial cells (fig1G–K). Many KL‐6 positive cells were observed in the superficial layer, while the immunoreactive cells were not detected in the subepithelial layer. Although KL‐6 immunopositive cells were detected in the basal layer of the pseudopterygium, superficial cells did not express KL‐6. The number of KL‐6 immunopositive epithelial cells was lower than those in pterygia and the normal conjunctiva. Table 1 summarises immunopositive rate of KL‐6.
Figure 1 Haematoxylin and eosin staining (A, G), and expression of KL‐6 (B–E, red) and Ki‐67 (F, L) in the human pterygium head (A–F) and body (G–L). KL‐6 immunoreactivity is detected in the pterygium tissue (B–D). At high magnification, immunoreactivity for KL‐6 is located in the cytoplasm and on the cell membrane of many epithelial cells (E). KL‐6 expression is not detected in superficial cells (E, arrows), nor in the basement membrane (C–E, arrows). Immunoreactivity for KL‐6 is strongly noted in pterygium body epithelium (H–J), especially in the cytoplasm (K). Nuclear immunoreactivity for Ki‐67 is detected in several pterygium epithelial cells (F, L). Green: nuclear staining with YO‐Pro‐1. Bar = 50 μm.
Table 1 Summary of KL‐6 immunopositive pattern in normal human conjunctiva, pterygia, and a pseudopterygium.
| Total (%) | Cytoplasmic/ circumferential membrane | Apical | |
|---|---|---|---|
| Normal conjunctiva | |||
| Superficial | 0 | 0 | 0 |
| Wing | 100 | 7.3 | 92.7 |
| Basal | 100 | 3.0 | 97.0 |
| Pterygium head | |||
| Superficial | 25 | 0 | 25.0 |
| Suprabasal | 100 | 9.4 | 90.6 |
| Basal | 90 | 5.0 | 85.0 |
| Pterygium body | |||
| Superficial | 83.3 | 35.7 | 47.6 |
| Suprabasal | 100 | 62.5 | 37.5 |
| Basal | 100 | 65.5 | 34.5 |
| Pseudopterygium | |||
| Superficial | 16.7 | 8.4 | 8.3 |
| Suprabasal | 58.2 | 9.5 | 48.7 |
| Basal | 65.5 | 3.4 | 62.1 |
Nuclear immunoreactivity for Ki‐67 was detected in each epithelium (fig1F, L). The number of Ki‐67‐positive nuclei was higher in pterygium head (labelling index: 13.6%) than that in the body (3.3%).
Comment
There was no significant difference in KL‐6 immunopositive rate of basal and suprabasal layers between pterygia and normal conjunctiva. This suggests that pterygia seem to show no obvious change in mucin secretion compared with normal conjunctiva. In contrast, KL‐6 was downregulated in the pseudopterygium, implicating advanced loss of the conjunctival epithelium's ability to produce mucin. Although it is sometimes hard to distinguish pteryiga from pseudopterygia histopathologically, pseudopterygia clearly differ from pterygia with regard to KL‐6 expression.
In this study, we showed the diversity of subcellular immunolocalisation of KL‐6 in pterygia and the normal conjunctiva. As recently demonstrated, the cytoplasm/circumferential membrane staining pattern of KL‐6 in colorectal carcinoma contributed to unfavourable prognosis when compared with apical membrane patterns.6 Moreover, the number of Ki‐67 positive nuclei was higher in the pterygium head than in the body, indicating that proliferation activity was high in the pterygium apex. Taken together, subcellular reactivity of KL‐6 in human pterygia might be correlated with pathobiological behaviour such as corneal invasion.
It has been demonstrated that pterygium body fibroblasts play an important part in the pathogenesis and development by expressing gene products.1,7,8 As recently reported, KL‐6 molecules had pro‐proliferative and anti‐apoptotic effects on lung fibroblasts,9 which are correlated with epithelial‐mesenchymal interactions in interstitial lung disease.9 The upregulation of KL‐6 expression might be associated with the proliferation of pterygium fibroblasts and invasion of the cornea.
Acknowledgements
This study was supported by a grant for research on sensory and communicative disorders from the Ministry of Health, Labor, and Welfare, and by grants in aid for Scientific Research from The Ministry of Education, Culture, Sports, Science, and Technology (MEXT), Japan.
References
- 1.Solomon A, Grueterich M, Li D Q.et al Overexpression of Insulin‐like growth factor‐binding protein‐2 in pterygium body fibroblasts. Invest Ophthalmol Vis Sci 200344573–580. [DOI] [PubMed] [Google Scholar]
- 2.Kohno N, Akiyama M, Kyoizumi S.et al Detection of soluble tumor‐associated antigens in sera and effusions using novel monoclonal antibodies, KL‐3 and KL‐6, against lung adenocarcinoma. Jpn J Clin Oncol 198818203–216. [PubMed] [Google Scholar]
- 3.Kohno N, Kyoizumi S, Awaya Y.et al New serum indicator of interstitial pneumonitis activity. Sialylated carbohydrate antigen KL‐6. Chest 19899668–73. [DOI] [PubMed] [Google Scholar]
- 4.Kitaichi N, Ariga T, Kase S.et al Usefulness of quantifying serum KL‐6 levels in the follow‐up of uveitic patients with sarcoidosis. Graefes Arch Clin Exp Ophthalmol 2006244433–437. [DOI] [PubMed] [Google Scholar]
- 5.Kitaichi N, Kotake S, Shibuya H.et al Increase of KL‐6 in sera of uveitis patients with sarcoidosis. Graefes Arch Clin Exp Ophthalmol 2003241879–883. [DOI] [PubMed] [Google Scholar]
- 6.Guo Q, Tang W, Inagaki Y.et al Clinical significance of subcellular localization of KL‐6 mucin in primary colorectal adenocarcinoma and metastatic tissues. World J Gastroenterol 20061254–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solomon A, Li D Q, Lee S B.et al Regulation of collagenase, stromelysin, and urokinase‐type plasminogen activator in primary pterygium body fibroblasts by inflammatory cytokines. Invest Ophthalmol Vis Sci 2000412154–2163. [PubMed] [Google Scholar]
- 8.Touhami A, Di Pascuale M A, Kawatika T.et al Characterisation of myofibroblasts in fibrovascular tissues of primary and recurrent pterygia. Br J Ophthalmol 200589269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohshimo S, Yokoyama A, Hattori N.et al KL‐6, a human MUC1 mucin, promotes proliferation and survival of lung fibroblasts. Biochem Biophys Res Commun 20053381845–1852. [DOI] [PubMed] [Google Scholar]