Abstract
Background/aim
Children with congenital microphthalmos are usually able to wear an eye prosthesis but the cosmetic aspect is determined by the size of the orbital volume deficiency. Instead of using a ball shaped standard hydrogel expander or a regular orbital implant, which would necessitate enucleation of the microphthalmic eye, this study investigates the feasibility of volume augmentation with injectable pellet expanders, as formerly suggested for acquired anophthalmos in adults only.
Method
The pellet expander is made from a self inflating hydrogel that takes up water by osmosis (dry state: length 8 mm, diameter 2 mm, volume 0.025 ml; in vitro hydrated state after around 1 day: length 15 mm, diameter 4 mm, volume 0.24 ml; swelling capacity: 9.6‐fold). This report concerns six patients (two girls and four boys) aged between 4 months and 42 months with unilateral microphthalmos who were treated by injection of 4–14 pellet expanders into the retrobulbar orbital tissue. Volume augmentation was 1–3.5 ml. The pellets were injected using a customised trocar and placed behind the microphthalmos directed into the intraconal space.
Results
The increasing orbital volume was noticeable within 2 days and was confirmed by ultrasonography and magnetic resonance imaging. The final result can be anticipated by the volume augmentation effect produced by the amount of saline solution injected in the orbital apex region. All patients were fitted with an artificial eye, which was subsequently enlarged every 3–5 months. Anophthalmic enophthalmos was fully compensated with this technique. No complications have been encountered to date.
Conclusions
Orbital volume augmentation with injectable self inflating hydrogel expander pellets is apparently a safe, quick, and minimally invasive technique for various indications in orbital reconstructive surgery—for example, to treat an enophthalmic appearance in microphthalmos and congenital or acquired anophthalmos.
Keywords: congenital microphthalmos, orbital volume deficiency, injectable expander, pellet expander, hydrogel, children
Congenital microphthalmos is more common than congenital anophthalmos and has a prevalence rate of between 1.2 and 1.8 per 10 000 births in white populations.1 It is assessed as being present if the age adjusted axial diameter is below the 95th centile.2 According to the classification proposed by Warburg, the condition described in this paper is simple total microphthalmos—visible globe when opening the lid fissure.2 All patients included in this series were without light perception in the microphthalmic eye.
Children with clinical anophthalmos or microphthalmos require prolonged and complicated socket management, and this is more difficult when the globe is not clinically apparent.3 Therapeutic options include the use of rigid conformers,4,5 low hydrophilic6 or, more recently, highly hydrophilic self expanding hydrogel expanders.7,8
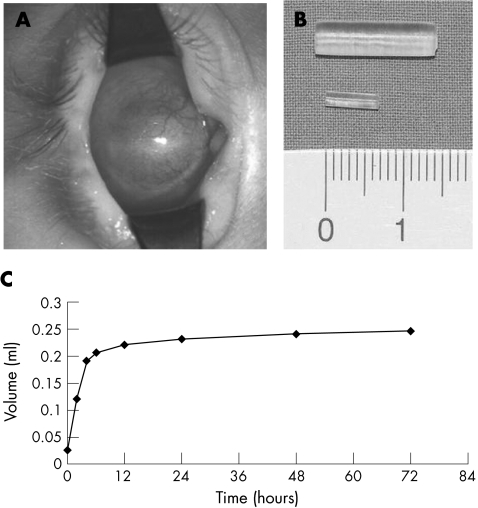
When the eyeball is reduced in volume it is deeply set in a small orbit. The palpebral fissure is usually narrowed, and in most cases there may be a fairly deep socket present with proper fornices (fig 1A). Under such circumstances it is much easier to fit an artificial eye.
Figure 1 (A) Right sided blind microphthalmos. Clinical picture: fornices well developed. (B) Pellet expanders, before (below) and after (above) in vitro swelling in 0.9% sodium chloride. (C) In vitro swelling curve.
In the case of a small microphthalmos with an axial length that differs significantly from the healthy side or from normal values, an orbital volume deficit results.3,9 The smaller the presentation of the microphthalmic eye, the more significant this becomes. While such an orbital volume deficit can be partially compensated with a prosthesis, the degree of adjustment from the patient's perspective is determined by the lid fissure width, which limits the size and shape of prosthesis that can be placed in the conjunctival sac. Therefore, even with the best fitting artificial eye, there may be some residual volume deficit that appears as enophthalmos. Consequently, there is a need for methods of orbital volume compensation.
Since 1997 our team in Rostock has been developing a novel therapeutic concept with highly hydrophilic self expanding hydrogel expanders for use in patients with congenital anophthalmos.8,10,11 This technique has recently been reviewed by Mazzoli et al7 and now appears to be well accepted. At the Congress of the American Society of Ophthalmic Plastic and Reconstructive Surgery (ASOPRS) in 2003, Li et al12 suggested using injectable hydrogel implants as a technical modification to achieve volume augmentation in adults with acquired anophthalmic enophthalmos.
To the best of our knowledge, this report is the first to describe the use of this technique in children with congenital microphthalmos.
Method
Ophthalmic evaluation
Ophthalmic evaluation must initially determine the presence or absence of any visual potential. Depending on the patient's age and degree of pathology, tests were used to assess for fixation, ability to follow moving objects or preferential looking. Visual evoked potentials (VEP) were additionally determined in all patients. Oculoplastic rehabilitation was only started once it was established that there was no visual potential in the microphthalmic eye.
Specific concerns for oculoplastic evaluation were first directed towards the microphthalmic socket in unilateral cases, but the status of the contralateral, probably healthy side, was examined very precisely to exclude any accompanying pathology.
Expander data
The pellet expander (fig 1B) is made of a highly hydrophilic hydrogel consisting of N‐vinyl pyrrolidone and methyl methacrylate (osmed GmbH, Ilmenau, Germany). It automatically takes up water by osmosis and is therefore self inflating. The amount of expansion (swelling capacity) can be engineered. In the dry state the pellet expander is 8 mm in length and 2 mm in diameter with a volume of 0.025 ml. In vitro, in the hydrated state after about 1 day (fig 1C), these dimensions increase to 15 mm in length and 4 mm in diameter, with a final volume of 0.24 ml. The swelling capacity is therefore 9.6‐fold.
Expander implantation
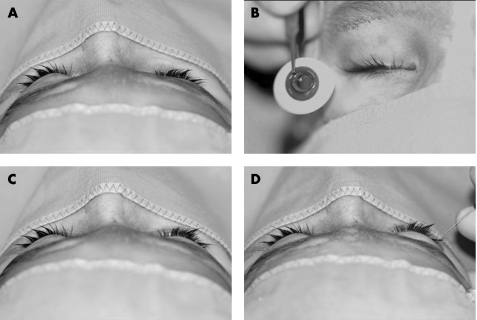
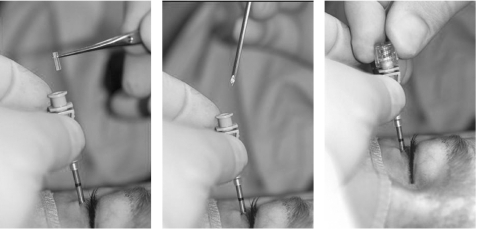
The first step was to insert a custom made, usually double walled glass prosthesis (fig 2). The size of the volume deficit was calculated by injecting sterile saline solution or local anaesthetic (fig 2). The fluid was injected through a standard retrobulbar needle until symmetry with the healthy side was achieved, as monitored using intraoperative Hertel measurements. The volume injected was divided by the potential final expander volume of 0.24 ml to yield the number of pellets needed for injection. The pellets were injected via the same cutaneous approach using a customised trocar and were placed behind the microphthalmos directed into the intraconal space (fig 3). The skin was closed with a single suture only.
Figure 2 Prosthesis. (A) Right sided enophthalmos due to congenital microphthalmos. (B) First prosthesis. (C) Prosthesis fitted, enophthalmos with prosthesis reduced but still significant. (D) Volume deficit calculation, symmetry achieved after 3.5 ml Xylocitin injection.
Figure 3 Expander injection. Trocar needle placed into the retrobulbar tissue in the intraconal space, pellet expander inserted, and pushed into the tissue.
Patients
To date, six patients with unilateral microphthalmos have been treated with injectable pellet expanders made from self inflating hydrogel (see table 1, figs 4 and 5). All patients were otherwise healthy and there was no family history of malformations.
Table 1 Patient data, volume augmentation with four (volume: 1 ml) to 14 (volume: 3.5 ml) pellets per patient, average volume 2.6 ml.
| Patient no | Sex | Side | Age first expander implantation (months) | Axial length (mm) RE/LE | Number of pellets implanted | Prosthesis fitted | Further pellets needed | Further prosthesis enlargement | Follow up (month) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | RE | 5 | 19/11 | 4 | yes | at age 7 month another 10 pellets implanted | every 2–3 months | 17 |
| 2 | F | RE | 7 | 10/22 | 7 | yes | – | every 4–5 months | 13 |
| 3 | M | RE | 8 | 8/21 | 8 | yes | – | every 2–3 months | 9 |
| 4 | M | LE | 8 | 21/12 | 10 | yes | – | 2 months later | 7 |
| 5 | M | RE | 4 | 8/22 | 10 | yes | – | 2 months later | 7 |
| 6 | F | RE | 42 | 14/23 | 14 | yes | – | planned | 5 |
| Average | M 4 | 4–42 | 8–14 | 4–14 | all | 1 case | 5–17 | ||
| F 2 | (12.33) | (10.5) | (10.5) | (9.7) |
RE, right eye; LE, left eye.
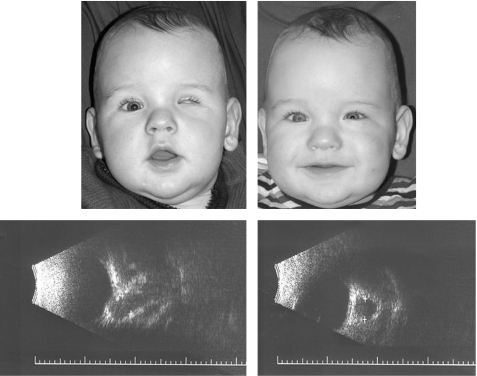
Figure 4 A 4 month old boy. Left sided microphthalmos, axial length 8 mm, before and after injection of 10 pellets (2.5 ml); postoperative ultrasound demonstrates retrobulbar expander placement.
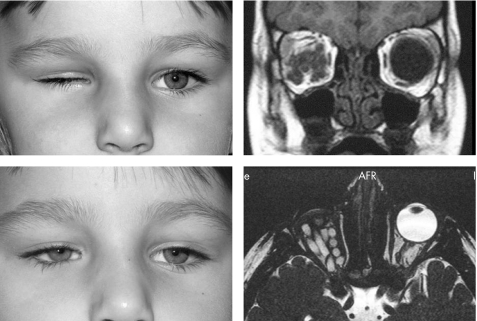
Figure 5 A 42 month old girl. Right sided microphthalmos, axial length 14 mm, before and after injection of 14 pellets (3.5 ml); postoperative magnetic resonance imaging.
Patient 1
A 5 month old boy with blind microphthalmos on the right side, no previous treatment. In March 2004 four pellets implanted and double walled glass prosthesis inserted; in May 2004 a further 10 pellets injected because of inadequate volume replacement; prosthesis was enlarged every 2–3 months thereafter.
Patient 2
A 7 month old girl with blind microphthalmos on the right side, no previous treatment. In July 2004 seven pellet expanders implanted and glass shell inserted; 3 months later the glass shell was replaced by a double walled glass prosthesis.
Patient 3
An 8 month old boy with blind microphthalmos on the right side, no previous treatment. In November 2004: eight pellet expanders injected and glass shell inserted, 2 months later the glass shell was replaced by a double walled glass prosthesis.
Patient 4
An 8 month old boy, the only patient in our series with microphthalmos on the left side. In January 2005 10 pellet expanders injected and glass shell fitted; 2 months later the glass shell was replaced by a custom made prosthesis.
Patient 5 (fig 4)
A 4 month old boy with blind microphthalmos on the right side. The patient had a healthy twin brother. PMMA conformer had been inserted previously, but no prosthesis. In February 2005 10 pellet expanders injected and glass shell fitted; 2 months later the glass shell was replaced by a custom made prosthesis.
Patient 6 (fig 5)
A 42 month old girl with blind microphthalmos on the right side; she and her twin sister were otherwise healthy. Conformers fitted previously on at least four occasions under general anaesthesia, but without lasting success. No prosthesis previously. In March 2005 glass prosthesis fitted and 14 pellet expanders injected; temporary tarsorrhaphy removed after 2 days.
All patients experienced improved orbital volume after injection. The main volume increase was noticeable within the first 24 hours and was completed within 2 days. This finding was confirmed by ultrasonography and magnetic resonance imaging (figs 4 and 5).
As far as we have been able to judge, these young patients experienced no significant pain during the expansion period. Two patients needed no specific medication for pain relief, three patients received one paracetamol suppository on the evening after implantation, and one patient received one paracetamol suppository daily for 3 days.
Preoperatively, all microphthalmic eyes showed some motility in all directions of gaze. Pellet implantation did not appear to affect motility in any case.
In each case the prosthesis was subsequently replaced with a larger size, as dictated by the clinical impression. So far no re‐injection has been necessary, except in the very first patient who received an injection of less than the calculated volume for safety reasons. To date, no patient has experienced expander prolapse or extrusion. No inflammatory signs or other side effects have been observed.
Discussion
The degree of globe deformity determines which oculoplastic rehabilitating techniques are appropriate for use. The critical first step is to rule out any visual potential in the microphthalmic globe. To cover an eye that has significant visual potential with an opaque prosthesis would obviously produce deprivational amblyopia. Endangering the globe with high pressures delivered by an expanding device is contraindicated even where visual acuity is limited to light perception.
The acceptable approach to prosthetic management of microphthalmic cases remains controversial, and no uniform strategy exists. Treatment may consist of observation only or in severe cases may involve surgical removal of the blind globe. In cases with an accompanying orbital cyst (not observed in the patients reported here) therapy is characterised by the surgical approach.13
Mustardé14 suggested complete excision of the rudimentary microphthalmic eye and the existing socket lining as a prelude to replacement with a skin lined cavity of suitable size. For this purpose he advocated using a skin graft, with the epithelial surface inside, wrapped around a conformer, which was fixed with external pins for several weeks. Despite the need for a complex surgical procedure, touching the conjunctiva often leads to significant shrinkage and may make it difficult or even impossible to fit a prosthesis.
Several clinical and experimental studies have confirmed that enucleation in childhood compromises orbital growth.15,16 The earlier enucleation is performed, the greater the reduction in orbital bone growth, especially if no orbital implant is used.15 It is believed that the growth of even a microphthalmic globe might stimulate orbital growth much more effectively than an artificial implant. Therefore preference should be given to therapy that avoids enucleation.
Most authors suggest treatment using increasingly larger conformers,17 but this method necessitates very intensive, sometimes weekly, treatment to gauge progress. The width of the lid fissure determines the size of the conformer or prosthesis, thus limiting the amount of volume that can be adjusted with a hard implant.
Dermis fat grafts have been proposed, as these have the advantage of being autologous and are able to enlarge as the child grows.18 Again removal of the microphthalmic globe would be necessary.
The concept of self inflating, highly hydrophilic hydrogels for use in patients with congenital anophthalmos originated in Rostock, Germany in 1997.19,20 Experience with this method has now been gained in almost 50 treated children.10,11
At the Congress of the American Society of Ophthalmic Plastic and Reconstructive Surgery (ASOPRS) in 2003, Li et al12 presented a modification of the shape, leaving the material itself unchanged, thus allowing expander injection for volume augmentation in adults with acquired anophthalmic enophthalmos. The first application of this approach in congenital microphthalmos was presented by our team at the Congress of the European Society of Ophthalmic Plastic and Reconstructive Surgery (ESOPRS) in 2004.21
Here we describe a series of six patients who have been followed for up to 17 months after injection. Orbital volume augmentation with injectable self inflating hydrogel expander pellets is a safe technique for the treatment of an enophthalmic appearance in congenital microphthalmos. The volume required ranged from 1 ml to 3.5 ml, with an average of 2.6 ml. The technique is minimally invasive and quick to perform. In the first patient ever treated a repeat injection was necessary because of under‐correction. From the second patient onwards the volume required has been calculated precisely. No complications have been observed to date, although longer follow up is necessary to assess the effect on orbital growth.
Pellet implantation did not appear to affect motility in the microphthalmic patients reported here. Although highly likely, it is not yet known whether the microphthalmos fitted with a thin prosthesis will be able to transmit some of this movement to the artificial eye in the long term.
In this regard the situation differs considerably from that in congenital anophthalmic sockets where parents have to be informed that even when the socket is well supported in volume terms, there will be virtually no prosthesis motility and in most cases no lid motility.11
The patients described in this paper were very young (age at first expander implantation: 4–8 months, with one exception, the patient aged 42 months); from the methodological standpoint precise motility measurements are therefore almost impossible. In addition to the age factor, the significantly reduced size of the globe makes it far more difficult to quantify motility.
Despite the specific application reported here, the technique may also be useful for a variety of indications in orbital reconstructive surgery—for example, in acquired anophthalmos, as mentioned by Li et al12 In particular, if a secondary procedure is needed, it is far easier to place pellet expanders than to insert an implant and there is no donor site morbidity as with a dermis fat graft, which is even more difficult to harvest and insert.
Admittedly, experiences with this material do not extend beyond 8 years—a circumstance regarded by Mazzoli et al7 as “the fly in this particular soup.” Those authors reviewed reported complications because of the lack of long term stability with MIRAGel, a material used in retinal surgery in the late 1970s but as far as is known, entirely unconnected with the hydrogel used in our patients. Our material differs chemically from MIRAGel in having a considerably more cross linked structure that renders it far more stable mechanically. In a material with a swelling capacity of 10, such as was used in our patients, it should also be remembered that only 10% of the implant expander consists of hydrogel—in our patients this corresponded only to 0.1–0.35 ml (average 0.26 ml)—while 90% is simple water. Nevertheless, careful follow up is mandatory to monitor for any side effects.
The guiding principle for congenital microphthalmic (blind) patients is conservative management with solid conformers/prostheses, avoiding surgery if at all possible. Preliminary results with the first six patients treated suggest that injectable hydrogel expanders are ideal to compensate for enophthalmos because of orbital volume deficiency in cases where this condition results in disturbing assymetry.
Abbreviations
ASOPRS - American Society of Ophthalmic Plastic and Reconstructive Surgery
ESOPRS - European Society of Ophthalmic Plastic and Reconstructive Surgery
VEP - visual evoked potentials
References
- 1.Stoll C, Alembik Y, Dott B.et al Epidemiology of congenital eye malformations in 131,760 consecutive births. Ophthalmic Paediatr Genet 199213179–186. [DOI] [PubMed] [Google Scholar]
- 2.Warburg M. Classification of microphthalmos and coloboma. J Med Genet 199330664–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krastinova D, Kelly M B, Mihaylova M. Surgical management of the anophthalmic orbit. Part 1: Congenital. Plast Reconstr Surg 2001108817–826. [DOI] [PubMed] [Google Scholar]
- 4.Collin J R, Moriarty P A. Management of the contracted socket. Trans Ophthalmol Soc UK 198210293–97. [PubMed] [Google Scholar]
- 5.Dootz G L. The ocularists' management of congenital microphthalmos and anophthalmos. Adv Ophthalmic Plast Reconstr Surg 1992941–56. [PubMed] [Google Scholar]
- 6.Downes R, Lavin M, Collin R. Hydrophilic expanders for the congenital anophthalmic socket. Adv Ophthalmic Plast Reconstr Surg 1992957–61. [PubMed] [Google Scholar]
- 7.Mazzoli R A, Raymond W R, Ainbinder D J.et al Use of self‐expanding, hydrophilic osmotic expanders (hydrogel) in the reconstruction of congenital clinical anophthalmos. Curr Opin Ophthalmol 200415426–431. [DOI] [PubMed] [Google Scholar]
- 8.Schittkowski M P, Gundlach K K, Guthoff R F. [Treatment of congenital clinical anophthalmos with high hydrophilic hydrogel expanders]. Ophthalmologe 2003100525–534. [DOI] [PubMed] [Google Scholar]
- 9.Schittkowski M P, Hingst V, Knaape A.et al [Orbital volume in congenital clinical anophthalmos]. Klin Monatsbl Augenheilkd 2004221898–903. [DOI] [PubMed] [Google Scholar]
- 10.Gundlach K K, Guthoff R F, Hingst V H.et al Expansion of the socket and orbit for congenital clinical anophthalmia. Plast Reconstr Surg 20051161214–1222. [DOI] [PubMed] [Google Scholar]
- 11.Schittkowski M P, Katowitz J A, Gundlach K K.et al Self‐inflating hydrogel expanders for the treatment of congenital anophthalmos . In: Guthoff RF, Katowitz JA, eds. Essentials in ophthalmology. oculoplastics and orbit 1st ed. Berlin: Springer, 2005205–221.
- 12.Li T G, McCann J D, Goldberg R A. Orbital volume augmentation in anophthalmic patients using injectable hydrogel implants. ASOPRS Abstracts 200391
- 13.Chaudhry I A, Arat Y O, Shamsi F A.et al Congenital microphthalmos with orbital cysts: distinct diagnostic features and management. Ophthal Plast Reconstr Surg 200420452–457. [DOI] [PubMed] [Google Scholar]
- 14.Mustardé J C. The eye socket. In: Repair and reconstruction in the orbital region. 2nd ed. Edinburgh, London, New York: Churchill Livingstone, 1980215–244.
- 15.Apt L, Isenberg S. Changes in orbital dimensions following enucleation. Arch Ophthalmol 197390393–395. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy R E. The effect of early enucleation on the orbit in animals and humans. Adv Ophthalmic Plast Reconstr Surg 199291–39. [PubMed] [Google Scholar]
- 17.McLean C J, Ragge N K, Jones R B.et al The management of orbital cysts associated with congenital microphthalmos and anophthalmos. Br J Ophthalmol 200387860–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Handler L F, Heher K L, Katowitz J A. Congenital and acquired anophthalmia. Curr Opin Ophthalmol 1994584–90. [PubMed] [Google Scholar]
- 19.Bacskulin A, Vogel M, Wiese K G.et al New osmotically active hydrogel expander for enlargement of the contracted anophthalmic socket. Graefes Arch Clin Exp Ophthalmol 200023824–27. [DOI] [PubMed] [Google Scholar]
- 20.Wiese K G, Vogel M, Guthoff R.et al Treatment of congenital anophthalmos with self‐inflating polymer expanders: a new method. J Craniomaxillofac Surg 19992772–76. [DOI] [PubMed] [Google Scholar]
- 21.Schittkowski M P, Guthoff R F. Injectable self‐inflating hydrogel pellet expanders for treatment of orbital volume deficiency in congenital microphthalmos—first experiences with a new technique. Abstracts of the 22nd ESOPRS 2004 [DOI] [PMC free article] [PubMed]