Abstract
Background/aims
Familial exudative vitreoretinopathy (FEVR) is an inherited blinding condition characterised by abnormal development of the retinal vasculature. FEVR has multiple modes of inheritance, and homozygous mutations in LRP5 have recently been reported as underlying the recessive form of this disease. The aim of this study was to examine LRP5 in a consanguineous recessive FEVR family and to clarify the eye and bone phenotype associated with recessive FEVR.
Methods
All family members were examined by slit lamp biomicroscopy and indirect ophthalmoscopy. Linkage to LRP5 was determined by genotyping microsatellite markers, constructing haplotypes and calculating lod scores. Mutation screening of LRP5 was performed by polymerase chain reaction amplification of genomic DNA followed by direct sequencing. Bone mineral density (BMD) was evaluated in all family members using dual energy x ray absorptiometry (DEXA).
Results
The clinical features observed in this family were consistent with a diagnosis of recessive FEVR. A homozygous LRP5 missense mutation, G550R, was identified in all affected individuals and all unaffected family members screened were heterozygous carriers of this mutation. Reduced BMD, hyaloid vasculature remnants, and nystagmus were features of the phenotype.
Conclusion
Recessive mutations in LRP5 can cause FEVR with reduced BMD and hyaloid vasculature remnants. Assessment of a patient with a provisional diagnosis of FEVR should therefore include investigation of BMD, with reduced levels suggestive of an underlying LRP5 mutation.
Keywords: familial exudative vitreoretinopathy, LRP5 , bone density, hyaloid vasculature
Familial exudative vitreoretinopathy (FEVR) is a Mendelian disease first described by Criswick and Schepens in 1969.1 The disease is characterised by bilateral deficient retinal vessel development, particularly in the temporal retinal periphery.1,2,3,4 This aspect of the phenotype is reported to be universal in affected individuals when examined by fundus fluorescein angiography,5 although it often causes no symptoms.3,5,6 The FEVR phenotype, however, is highly variable because many individuals develop a myriad of retinal abnormalities triggered by ischaemia in the avascular retina. These include retinal neovascularisation, retinal traction, and vascular exudates, ultimately leading to retinal detachment.2,3
FEVR can be inherited as either an autosomal dominant (MIM133780), recessive (MIM601813), or X linked trait (MIM305390), with autosomal dominant being the commonest mode of inheritance. To date, mutations causing dominant disease have been identified in two genes; frizzled‐4 (FZD4) on chromosome 11q14 and low density lipoprotein receptor related protein 5 (LRP5) nearby on chromosome 11q13.7,8 Both of these genes encode receptors for Wnt proteins thus implicating this signalling pathway in this disease. At least two further dominant FEVR genes remain to be identified; EVR3 on chromosome 11p12–13 and a further locus that is currently being mapped.9,10
Mutations underlying X linked FEVR have been identified in the Norrie disease gene (NDP) on chromosome Xp11.11NDP encodes Norrin which has been shown to function as a high affinity ligand for FZD4. Similar to Wnt proteins, its binding to FZD4 also activates the canonical β catenin pathway in the presence of LRP5.12 Thus to date, all three FEVR genes identified encode proteins that appear to function in the same Wnt/Norrin signalling pathway.12
Following the discovery that heterozygous LRP5 mutations underlie dominant FEVR,8LRP5 was also identified as a recessive FEVR gene after homozygous missense mutations were identified in three well characterised recessive FEVR families.13 Surprisingly, none of the affected members of these three families were reported to have any skeletal abnormalities despite a catalogue of evidence linking LRP5 mutations to bone disorders. Recessive LRP5 mutations are known to underlie osteoporosis pseudoglioma syndrome (OPPG) (MIM259770), a disorder characterised by very low bone mass and congenital or infancy onset blindness.14 Similarly, reduced bone mass has been reported in the heterozygous mutation carriers in OPPG families and in FEVR patients with dominant LRP5 mutations.8,15,16 Likewise, heterozygous LRP5 mutations have been reported in children with primary osteoporosis and many population studies suggest that polymorphisms within LRP5 contribute to the population variance in bone mineral density (BMD).17,18 Dominant missense mutations, thought to result in a gain of function of LRP5, have also been described in patients with high bone mass disorders.19,20,21
In this study, we identify a novel homozygous missense LRP5 mutation in a consanguineous recessive FEVR family and fully characterise the family's phenotype showing that reduced BMD is a feature of recessive LRP5‐FEVR.
Materials and methods
Patients
Three affected siblings were identified from a UK family of Pakistani origin in which the parents were first cousins. Detailed clinical examination of six family members was undertaken and blood samples were collected with informed consent. Ethical approval was provided by the Leeds Teaching Hospitals Trust research ethics committee.
Ophthalmic examination
The eye examinations of all patients included ascertainment of best corrected visual acuity, external examination, motility evaluation, slit lamp biomicroscopy, and indirect ophthalmoscopy. The Zeiss SFA450IR fundus camera was used to photograph the fundus.
Bone density examination
All family members underwent evaluation of serum 25‐hydroxycholecalciferol, serum calcium, serum phosphate, serum alkaline phosphatase and serum parathyroid hormone levels to exclude any biochemical disturbance of bone metabolism. Family members also underwent standard DEXA bone scanning to measure BMD using the GE Lunar Prodigy DEXA densitometer. T scores (number of standard deviations from the mean derived from healthy young sex matched adults) are the usual format for expressing BMD in an adult. Using an adult population as the standard is not appropriate for children, so instead Z scores (standard deviations from the mean derived from an age, sex, and racially matched population) are generated. In addition small body size can produce a spuriously low BMD and so calculation of a volumetric BMD Z score (corrected for body size) is also useful.22 In this pedigree a Z score was generated for all family members by imaging the spine, while the affected children also underwent total body imaging to allow generation of a volumetric BMD Z score.
Linkage analysis
Genotyping was performed using fluorescently tagged microsatellite markers. Polymerase chain reactions (PCR) were carried out as previously described.10 Following amplification, PCR products were resolved using a MegaBACE 500 DNA sequencer and analysed using Fragment Profiler 1.2 software (GE Healthcare). Linkage analysis was performed under the assumption of a recessive model with 100% penetrance and 0.001 frequency of the disease allele. The phenocopy rate was set at 0.1%. Multipoint linkage analyses were performed using LinkMap.23 The UCSC Genome Browser (http://genome.cse.ucsc.edu) and DeCODE genetic map were used to determine the marker order and genetic distances.24 The analysis was performed twice using either equal marker allele frequencies or marker allele frequencies obtained from the CEPH genotype database browser (www.cephb.fr).
Mutation analysis
The exons and flanking intronic sequences of LRP5 were PCR amplified using the primer sequences and conditions previously reported.14 Both the forward and reverse strands of the PCR products were directly sequenced on a MegaBACE 500 DNA sequencer using the DYEnamic ET terminator kit (GE Healthcare, UK). Sequencing reactions were set up according to the manufacturer's instructions with the same primers used to amplify the PCR products.
GenBank accession numbers
Protein sequences for LRP5 homologue alignment figure: human NP_002326, Pan troglodytes XP_508605, Canis familiaris XP_533209, Mus musculus NP_032539, Gallus gallus NP_001012915, Xenopus laevis AAN09806, Tetraodon nigroviridis CAF90682, Anopheles gambiae str XP_320740 and Drosophila melanogaster AAF91072.
Results
Clinical evaluation
The family studied is shown in figure 1. The proband (IV:1) and two younger affected siblings (IV:3 and IV:4) all presented within the first year of life with a provisional diagnosis of congenital nystagmus but upon detailed examination all were found to have classic signs of FEVR. All were born at full term except IV:3 who was born at 38 weeks' gestation weighing 7 lb (3.2 kg).
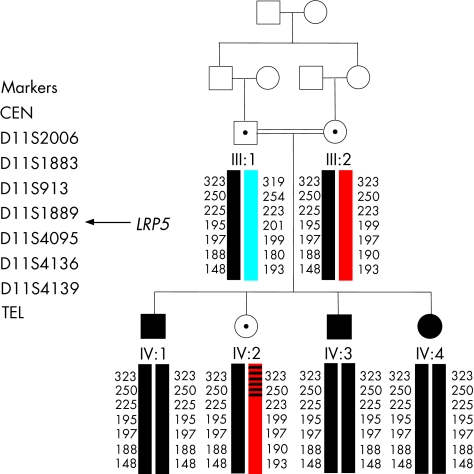
Figure 1 Pedigree of the autosomal recessive FEVR family analysed in this study. Individuals represented by solid symbols are confirmed as affected by clinical examination. Haplotypes spanning LRP5 are shown below each symbol. Each different haplotype block is represented by a different colour with the disease haplotype in black.
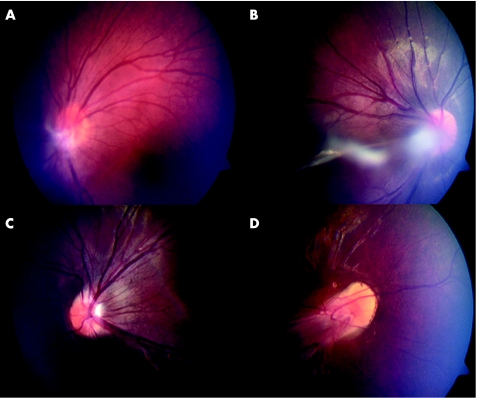
The proband (IV:1) is a 17 year old male who first presented with congenital esotropia and nystagmus shortly after birth. Retinal examination under anaesthesia (EUA) revealed bilateral peripheral retinal avascularity with extensive retinal exudates. Both eyes displayed glial remnants at the optic disc, with the left eye also showing a persistent hyaloid artery remnant projecting into the vitreous (fig 2A). Since age 16 his vision has remained at 6/12 bilaterally.
Figure 2 Clinical pictures of family members showing classic features of FEVR. (A) Fundus photograph of the proband's (IV:1) left eye taken at age 17. White glial tisssue is visible at the disc with the suggestion of a hyaloid artery remnant running forward into the vitreous. (B, C) Fundus photographs of IV:3 taken at age 12. In the right eye (B) a strand within the vitreous associated with glial tissue appears to obscure the posterior pole. In the left eye (C) there is slight straightening of the temporal arcades. (D) Fundus photograph of the right eye of IV:4 taken at age 10. Extensive vitreoretinal traction is seen distorting the retinal vessels emerging from the disc and producing a “dragged disc” appearance.
Patient IV:3 is a 12 year old male. During an EUA at age 2, he showed slight dragging of vessels at the optic disc bilaterally with retinal exudates (fig 2B and C). In the right eye a hyaloid artery remnant was seen extending from the disc to the posterior lens surface. At age 6 he had a sudden reduction of vision in his right eye due to vitreous haemorrhage. By 10 years of age his vision had stabilised at 6/60 in his right eye and 6/9 in the left. He was recorded as having a low hypermetropic refraction (+2.50 dioptres).
Patient IV:4 is a 10 year old female. EUA within her first year revealed dragged discs bilaterally. At age 4 she had developed preretinal fibrosis leading to a total retinal detachment in the left eye with acuities of 3/60 in the right eye (fig 2D) and hand movements only in the left. Her vision has remained stable at this level subsequently. She was documented as demonstrating a low hypermetropic refraction (+3.50DS; aged 2 years). She had previously undergone computed tomography neuroimaging as an infant because of poor muscle tone but no abnormality was reported.
Retinal examination was deemed normal in the remaining family members examined (III:1, III2, and IV:2).
LRP5 analysis
The severe phenotype, early age of disease onset, and consanguinity observed in this family suggested a recessive mode of inheritance. Analysis therefore focused on LRP5 and seven microsatellite markers surrounding this gene were genotyped in family members. Markers and distances used are as follows; 11ptel ‐ 65‐cM ‐ D11S2006 ‐ 4.07‐cM ‐ D11S1883 ‐ 2.52‐cM ‐ D11S913 ‐ 0.65‐cM ‐ D11S1889 ‐ 2.83‐cM/LRP5 ‐ D11S4095 ‐ 0.66‐cM ‐ D11S4136 ‐ 1.56‐cM ‐ D11S4139 ‐ 75‐cM ‐ 11qtel. Each family member's haplotype obtained from these markers is shown in figure 1. Multipoint linkage analysis with markers D11S913, D11S1889, and D11S4136 showed statistically significant evidence for linkage to LRP5 (lod score 2.3 using equal allele frequencies and 2.52 using CEPH allele frequencies), suggesting that LRP5 mutations were responsible for the FEVR seen in this family.
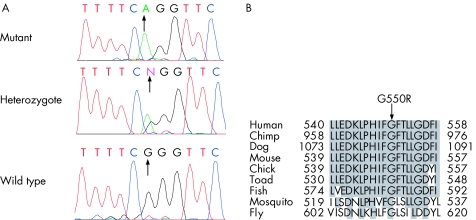
Mutation screening revealed a novel homozygous recessive LRP5 missense mutation in all three affected siblings in exon 8, c1648G>A (G550R) (fig 3A). Mother, father, and the unaffected sibling were heterozygous for this sequence change and were therefore assigned carrier status. The presence of this mutation was excluded in 280 ethnically matched control chromosomes and a further 120 white control chromosomes. We also checked the mutated amino acid for conservation within homologues of LRP5 from other species (fig 3B). The glycine at position 550 in LRP5 was fully conserved in all species for which sequence is available.
Figure 3 LRP5 mutation c1648G>A identified in recessive FEVR family. (A) Sequence trace of mutation in IV:1 (mutant), III:1 (heterozygote), and control individual (wild type). (B) Protein sequence alignment of human LRP5 with homologues from other species: Pan troglodytes (chimp), Canis familiaris (dog), Mus musculus (mouse), Gallus gallus (chick), Xenopus laevis (toad), Tetraodon nigroviridis (fish), Anopheles gambiae str (mosquito), and Drosophila melanogaster (fly). Only 20 amino acid residues surrounding each mutation are shown. Conserved amino acid residues are highlighted. G550R affects a fully conserved amino acid.
Bone density examination
Prompted by the association of LRP5 mutations with abnormalities of bone density, the family members underwent a full bone examination. Medical records showed that the younger brother (IV:3) had already sustained several lower limb fractures from minor falls, while the proband (IV:1) had previously been seen in infancy with abnormal curvature of both legs. His mother had been informed he had “bone thinning” but no further action was taken. The affected sister (IV:4) had no history of fractures. Serum bone biochemistry was within the normal range for all affected siblings.
The results of DEXA analysis are presented in table 1. T scores are expressed as standard deviations compared to the mean bone density of a 30 year old female population; as such they are not usually calculated for children. Z scores are similarly expressed as standard deviations compared to the mean of an age, sex, and racially matched population.22 A volumetric Z score is calculated in the same manner as a Z score but with a correction for body size; it is the most useful score in children. For each score a high negative value implies a greater deviation from the mean and hence a low bone density.
Table 1 DEXA results for family members.
| Patient | Spine Z score* | Neck of femur Z score* | Volumetric BMD Z score | Clinical status FEVR | Mutation status |
|---|---|---|---|---|---|
| IV:1 | −4.08 | not calculated | −2.1 | affected | homozygous mutation |
| IV:3 | −1.2 | not calculated | −1.6 | affected | homozygous mutation |
| IV:4 | −2.5 | not calculated | −2.5 | affected | homozygous mutation |
| III:2 | −0.80 (−0.80) | 1.20 (1.10) | not calculated | no cardinal features | heterozygote |
| III:1 | −1.20 (−1.20) | −0.20 (−0.50) | not calculated | no cardinal features | heterozygote |
| IV:2 | 0.40 | not calculated | not calculated | no cardinal features | heterozygote |
*T score in parentheses where calculated.
In table 1, BMD is presented as a Z score for the affected siblings. In order to allow comparison between family members, BMD is also presented as a Z score for the unaffected daughter and parents. In addition a volumetric Z score is presented for the younger family members. All three children affected with the ocular phenotype showed reduced bone mass; IV:1 and IV:3 are osteopenic while IV:4 is osteoporotic. The two youngest children have been commenced on Pamidronate therapy by a paediatric endocrinologist. IV:1 awaits assessment by an adult endocrinologist.
Discussion
We have described a consanguineous recessive FEVR pedigree with an underlying mutation within LRP5. Clinically, the three affected family members displayed a retinal phenotype typical of FEVR, including avascularity of a large portion of the peripheral retina, exudates, reduced visual acuity, and traction of the vessels emanating from the optic disc.3,4 Unusually, all affected individuals presented within the first year of life with nystagmus, an uncommon feature in FEVR, although rare reports have been described in recessive and X linked families.25,26,27
DEXA bone scan analysis of the affected siblings showed reduced bone mass suggestive of osteopenia in the two males and osteoporosis in the affected female. This is the first definitive report of reduced bone mass density associated with recessive FEVR. Previously reported recessive FEVR families harbouring LRP5 mutations showed no signs of reduced bone mass although diagnostic examinations were not performed.13 Reduced BMD has recently been reported in two simplex FEVR patients with heterozygous compound mutations within LRP5 suggestive of recessive FEVR. However, parents of these cases also showed reduced BMD and in one instance a parent also showed signs of mild FEVR when examined by fluorescein angiography, so a definite recessive mode of inheritance cannot be determined.16 This study therefore adds to the mounting evidence for bone density abnormalities as a consequence of LRP5 mutations.8,16 As such, an inquiry about bone defects/multiple fractures should form part of the systems review when taking a history from a patient with a provisional diagnosis of dominant or recessive FEVR.
An interesting feature observed in the two affected males in this pedigree was glial tissue at the disc reminiscent of Bergmeister's papillae (a posterior remnant of the hyaloid artery at the optic disc) along with more extensive hyaloid artery remnants. Similar remnants were reported in the recessive FEVR pedigree described by De Crecchio and colleagues, who observed vitreous strands connected to the lens and retrolental glial tissue in two affected sisters.25 Abnormalities of the hyaloid vasculature have rarely been reported in FEVR, with only two cases of persistent hyperplastic primary vitreous (PHPV) having been described in dominant and X linked families.28,29 However, despite the few cases reported to date, evidence now suggests that abnormal regression of the hyaloid vasculature during embryonic development (which is thought to underlie both PHPV and hyaloid remnants), is part of the pathogenesis of FEVR. Firstly, PHPV is reported in the mildly affected eyes of patients with OPPG and in a number of cases of Norrie's disease.29,30,31 Given that both of these disorders are allelic with FEVR, we may consider them to be part of the same clinical spectrum resulting from defects in the same developmental pathway. Secondly, defects in the regression of the hyaloid vasculature have been reported in mice with targeted disruptions of NDP and LRP5, further highlighting a role for these proteins in this process.32,33
The mutation identified in this family was a non‐conservative substitution changing a highly conserved glycine to an arginine within the second extracellular β propeller domain of LRP5. This is the same location previously reported to harbour missense mutations responsible for recessive FEVR (R570Q) and OPPG (R570W).13,14 At the moment there appears to be no phenotype‐genotype correlation between different LRP5 mutations and the disease severity observed. The mutation described in this report appears similar in location and conservation to many of the missense mutations reported to cause OPPG, but the milder phenotype indicates that this mutation is less pathogenic.29 Functional studies or animal studies will be needed to fully understand the nature of these mutations.
In summary, reduced bone density may be seen in association with recessive FEVR as a result of mutations in the LRP5 gene. It is therefore useful to ask about bone abnormalities and fracture when assessing any new patient with FEVR. Such an inquiry is particularly relevant if recessive inheritance is suspected or if an early onset of the phenotype is suggested by severe disease or nystagmus.
Acknowledgements
We thank the members of the FEVR family for their help with this study. The financial support of the Royal Society (CT is a Royal Society University Research Fellow), the Wellcome Trust (grants 069718/Z/02 and 073477/Z/03), and Yorkshire Eye Research is gratefully acknowledged.
Abbreviations
BMD - bone mineral density
DEXA - dual energy x ray absorptiometry
EUA - examination under anaesthesia
FEVR - familial exudative vitreoretinopathy
OPPG - osteoporosis pseudoglioma syndrome
PCR - polymerase chain reaction
PHPV - persistent hyperplastic primary vitreous
Footnotes
The authors have no competing interests.
References
- 1.Criswick V G, Schepens C L. Familial exudative vitreoretinopathy. Am J Ophthalmol 196968578–594. [DOI] [PubMed] [Google Scholar]
- 2.Miyakubo H, Hashimoto K, Miyakubo S. Retinal vascular pattern in familial exudative vitreoretinopathy. Ophthalmology 1984911524–1530. [DOI] [PubMed] [Google Scholar]
- 3.Benson W E. Familial exudative vitreoretinopathy. Trans Am Ophthalmol Soc 199593473–521. [PMC free article] [PubMed] [Google Scholar]
- 4.Van Nouhuys C E. Dominant exudative vitreoretinopathy and other vascular developmental disorders of the peripheral retina. Doc Ophthalmol 1982541–414. [DOI] [PubMed] [Google Scholar]
- 5.Ober R R, Bird A C, Hamilton A M.et al Autosomal dominant exudative vitreoretinopathy. Br J Ophthalmol 198464112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nijhuis F A, Deutman A F, Aan de Kerk A L. Fluorescein angiography in mild stages of dominant exudative vitreoretinopathy. Mod Probl Ophthalmol 197920107–114. [PubMed] [Google Scholar]
- 7.Robitaille J, MacDonald M L E, Kaykaset al Mutant frizzled‐4 disrupts retinal angiogenesis in familial exudative vitreoretinopathy. Nat Genet 200232326–330. [DOI] [PubMed] [Google Scholar]
- 8.Toomes C, Bottomley H M, Jackson R M.et al Mutations in LRP5 or FZD4 underlie the common FEVR locus on chromosome 11q. Am J Hum Genet 200474721–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Downey L M, Keen T J, Roberts E.et al A new locus for autosomal dominant familial exudative vitreoretinopathy maps to chromosome 11p12–13. Am J Hum Genet 200168778–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toomes C, Downey L M, Bottomley H M.et al Further evidence of genetic heterogeneity in familial exudative vitreoretinopathy; exclusion of EVR1, EVR3 and EVR4 in a large autosomal dominant pedigree. Br J Ophthalmol 200589194–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Z ‐ Y, Battinelli E M, Fielder A.et al A mutation in the Norrie disease gene (NDP) associated with X‐linked familial exudative vitreoretinopathy. Nat Genet 19935180–183. [DOI] [PubMed] [Google Scholar]
- 12.Xu Q, Wang Y, Dabdoub A.et al Vascular development in the retina and inner ear: control by Norrin and Frizzled‐4, a high‐affinity ligand‐receptor pair. Cell 2004116883–895. [DOI] [PubMed] [Google Scholar]
- 13.Jiao X, Ventruto V, Trese M T.et al Autosomal recessive familial exudative vitreoretinopathy is associated with mutations in LRP5. Am J Hum Genet 200475878–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gong Y, Slee R B, Fukai N.et al LDL receptor‐related protein 5 (LRP5) affects bone accrual and eye development. Cell 2001107513–523. [DOI] [PubMed] [Google Scholar]
- 15.Lev D, Binson I, Foldes J.et al Decreased bone density in carriers and patients of an Israeli family with the osteoporosis‐pseudoglioma syndrome. Isr Med Assoc J 20035419–421. [PubMed] [Google Scholar]
- 16.Qin M, Hayashi H, Oshima K.et al Complexity of the genotype‐phenotype correlation in familial exudative vitreoretinopathy with mutations in the LRP5 and/or FZD4 genes. Hum Mutat 200526104–112. [DOI] [PubMed] [Google Scholar]
- 17.Hartikka H, Makitie O, Mannikko M.et al Heterozygous mutations in the LDL receptor‐related protein 5 (LRP5) gene are associated with primary osteoporosis in children. J Bone Miner Res 200520783–789. [DOI] [PubMed] [Google Scholar]
- 18.Ferrari S L, Deutsch S, Antonarakis S E. Pathogenic mutations and polymorphisms in the lipoprotein receptor‐related protein 5 reveal a new biological pathway for the control of bone mass. Curr Opin Lipidol 200516207–214. [DOI] [PubMed] [Google Scholar]
- 19.Boyden L M, Mao J, Belsky J.et al High bone density due to a mutation in LDL‐receptor‐related protein 5.N Engl J Med 20023461513–1521. [DOI] [PubMed] [Google Scholar]
- 20.Little R D, Carulli J P, Del Mastro R G.et al A mutation in the LDL receptor‐related protein 5 gene results in the autosomal dominant high‐bone‐mass trait. Am J Hum Genet 20027011–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Wesenbeeck L, Cleiren E, Gram J.et al Six novel missense mutations in the LDL receptor‐related protein 5 (LRP5) gene in different conditions with an increased bone density. Am J Hum Genet 200372763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fewtrell M S. Bone densitometry in children assessed by dual X ray absorptiometry: uses and pitfalls. Arch Dis Child 200388795–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lathrop G M, Lalouel J M, Julier C.et al Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sci USA 1984813443–3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kong A, Gudbjartsson D F, Sainz J.et al A high‐resolution recombination map of the human genome. Nat Genet 200231241–247. [DOI] [PubMed] [Google Scholar]
- 25.De Crecchio G, Simonelli F, Nunziata G.et al Autosomal recessive familial exudative vitreoretinopathy: evidence for genetic heterogeneity. Clin Genet 199854315–320. [DOI] [PubMed] [Google Scholar]
- 26.Dudgeon J. Familial exudative vitreo‐retinopathy. Trans Ophthalmol Soc UK 19799945–49. [PubMed] [Google Scholar]
- 27.Fullwood P, Jones J, Bundey S.et al X linked exudative vitreoretinopathy: clinical features and genetic linkage analysis. Br J Ophthalmol 199377168–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riveiro‐Alvarez R, Trujillo‐Tiebas M J, Gimenez‐Pardo A.et al Genotype‐phenotype variations in five Spanish families with Norrie disease or X‐linked FEVR. Mol Vis 200511705–712. [PubMed] [Google Scholar]
- 29.Chang‐Godinich A, Paysse E A, Coats D K.et al Familial exudative vitreoretinopathy mimicking persistent hyperplastic primary vitreous. Am J Ophthalmol 1999127469–471. [DOI] [PubMed] [Google Scholar]
- 30.Ai M, Heeger S, Bartels C F.et al Clinical and molecular findings in osteoporosis‐pseudoglioma syndrome. Am J Hum Genet 200577741–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zaremba J, Feil S, Juszko J.et al Intrafamilial variability of the ocular phenotype in a Polish family with a missense mutation (A63D) in the Norrie disease gene. Ophthalmic Genet 199819157–164. [DOI] [PubMed] [Google Scholar]
- 32.LaRussa F, Wesson M D. Norrie's disease vs PHPV: one family's dilemma. J Am Optom Assoc 199263404–408. [PubMed] [Google Scholar]
- 33.Luhmann U F, Lin J, Acar N.et al Role of the Norrie disease pseudoglioma gene in sprouting angiogenesis during development of the retinal vasculature. Invest Ophthalmol Vis Sci 2005463372–3382. [DOI] [PubMed] [Google Scholar]
- 34.Kato M, Patel M S, Levasseur R.et al Cbfa1‐independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol 2002157303–314. [DOI] [PMC free article] [PubMed] [Google Scholar]