Abstract
Aims
To evaluate the anatomical outcomes, safety and functional effectiveness of surgical embolus removal in retinal artery occlusion (RAO).
Methods
Prospective study of seven patients with RAO of <36 h duration. All eyes underwent pars plana vitrectomy and a longitudinal incision of the anterior wall of the occluded arteriole in an attempt to remove the embolus. Outcome measures included visual acuity and arteriolar reperfusion, as evaluated with fluorescein angiography.
Results
Surgical removal of the embolus was achieved in six of the seven (87.5%) patients, visual acuity improved from a median of 20/400 (range: hand movements 20/25) to 20/40 (range: hand movements 20/25), and reperfusion of the occluded vessel was angiographically confirmed in four of the six patients in whom the embolus was successfully removed.
Conclusion
Surgical removal of retinal arterial emboli seems to be an effective and safe treatment for RAO, but a randomised and controlled clinical trial will be necessary to establish an evidence base for the role, if any, of this intervention.
Retinal artery occlusion (RAO) is a potentially devastating visual disorder, usually caused by blockage of a vessel by emboli or atheroma.1 The emboli, which are visible in 20–40% of eyes,2 mainly originate in the carotid arteries (74.5%) and are comprised of cholesterol.3 Fibrin‐platelet emboli (15.5%) and calcific emboli from the cardiac valves (10.5%) are also relatively frequent,3 whereas emboli caused by corticosteroid use,4 cardiac myxoma5 and intravenous drug misuse6 are uncommon. The site of the pathological process determines whether the central retinal artery (lamina cribrosa), a branch retinal artery or the cilioretinal artery will be affected. Experimental studies have shown that irreversible retinal damage occurs by 240 min after central RAO.7 Numerous treatment approaches have been attempted to improve vision in eyes with RAO, but none has proved particularly effective.8,9,10,11,12,13,14,15,16
In 1990, Peyman and Gremillion17 surgically removed one embolus in a patient with branch RAO of 60 h duration, with a visual acuity improvement to 20/200. The purpose of this study was to assess the anatomical outcome, safety and functional effectiveness of surgical embolus removal in seven consecutive patients with RAO.
Materials and methods
A prospective, interventional study was designed to treat seven patients with RAO. Inclusion criteria were duration <36 h, sudden decrease in visual acuity or visual field defect in the area of the obstructed vessel, absence of perfusion or marked perfusion delay in the affected area on fluorescein angiography, and visible embolus. Five patients had temporal branch RAO, one patient had hemispheric artery occlusion and one patient had central RAO.
Patients were fully informed of all aspects of the procedure, and all provided written informed consent. The study was approved by the hospital ethics committee.
Patient data included age, sex, affected eye, time from onset, visual acuity as measured by Early Treatment Diabetic Retinopathy Study charts, vascular risk factors, postoperative decrease in retinal whitening and arteriole reperfusion on fluorescein angiography, and changes in retinal thickness, as measured by optical coherence tomography (OCT 3; Humphrey model 2000; Humphrey Instruments, Dublin, California, USA). All tests were carried out preoperatively, and at 48 h, 1 week, 1 month and 6 months after surgery. Preoperative scotoma was evaluated by a Humphrey visual field analyser (model 2001; Humphrey Instruments).
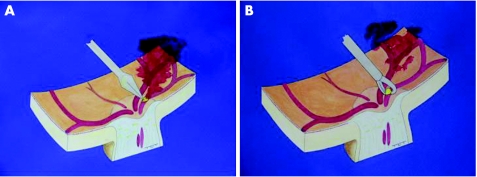
The same surgeon (JG‐A) carried out all the procedures. Standard three‐port, 20‐gauge pars plana vitrectomy was undertaken, and the posterior hyaloid was dissected from the retina under active aspiration. A longitudinal incision was made adjacent to the embolus in the anterior wall of the arteriole with a 25‐gauge microvitreoretinal blade (fig 1A). When bleeding was observed, the intraocular pressure was increased to 60–70 mm Hg and a silicone‐tipped cannula was used to remove intravitreal blood. If the embolus did not spontaneously pass through the wall incision, Tano asymmetrical vitreoretinal forceps (Synergetics, St Charles, Missouri, USA) were used to express it from the vessel (fig 1B). The arteriole was left unsutured and vasospasm closed the incision.
Figure 1 (A) A microvitreoretinal blade is used to penetrate the anterior arterial wall adjacent to the embolus, and a longitudinal incision is made. (B) Embolus expression and removal with microvitreoretinal forceps.
Results
The study sample included five men and two women of median age 65 years. Table 1 lists the demographic data of the patients.
Table 1 Demographic data of the patients.
| Patient no | Age (years), sex | Eye | Medical history | Time of evolution | Affected area | Preop BCVA | 48 h BCVA | Final BCVA | Preop OCT (μm) | Postop OCT (μm) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 62, F | Right | Cardiac valve disease | 22 | Inferotemporal | cf | 20/100 | 20/30 | 358 | 221 |
| 2 | 65, M | Right | Systemic hypertension | 4 | superohemispheric | hm | 20/200 | 20/50 | 325 | 243 |
| 3 | 62, M | Right | Carotid plaques | 19 | Inferotemporal | 20/200 | 20/100 | 20/25 | 393 | 225 |
| 4 | 63, M | Left | Systemic hypertension | 29 | CRAO | hm | hm | hm | 405 | 150 |
| 5 | 64, F | Right | Systemic hypertension | 33 | Superotemporal | 20/25 | 20/30 | 20/25 | 223 | 227 |
| 6 | 76, M | Left | Carotid plaques | 28 | Inferotemporal | 20/200 | 20/200 | 20/200 | 354 | 180 |
| 7 | 63, M | Left | Cardiac valve disease | 12 | Inferotemporal | 20/400 | 20/100 | 20/80 | 365 | 251 |
BCVA, best‐corrected visual acuity; cf, counting fingers; CRAO, central retinal vein occlusion; F, female; hm, hand movements; M, male; OCT, optical coherence tomography; postop, postoperative; preop, preoperative.
Preoperative best‐corrected visual acuity ranged from hand movements to 20/25 (median 20/400 (interquartile range (IQR) 20/250)). Time from onset to surgery ranged from 4 to 33 h (median 22 h). The embolus was visible in all patients, and there was an absence of filling or a substantial filling delay in the obstructed vessel on fluorescein angiography. Complete scotoma corresponding to the ischaemic area was observed in all patients. Median preoperative macular thickness was 358 μm (IQR 68).
The slight to moderate bleeding from the artery that occurred during wall dissection was controlled by increasing the intraocular pressure to 70 mm Hg when required. The initial incision had to be enlarged in all patients. In four patients, vitreoretinal forceps were required to express the embolus through the incision. Surgical embolus removal was achieved in six (87.5%) patients. Owing to its large size, the embolus could not be removed in the patient with central RAO.
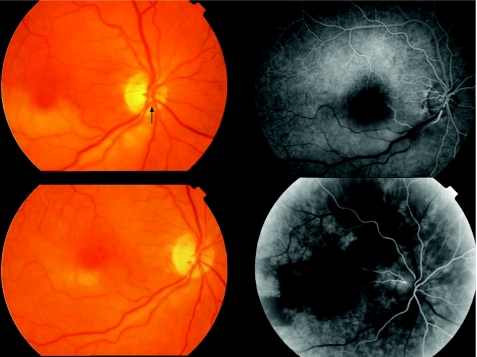
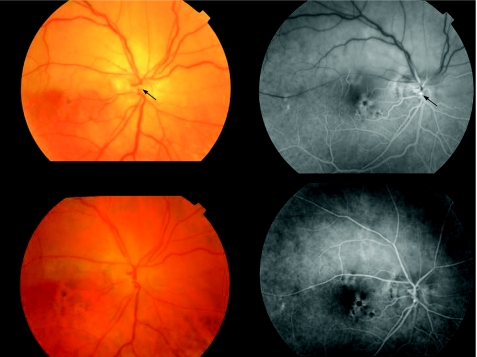
Slight vitreous haemorrhage in two (28.6%) patients in the early postoperative period cleared spontaneously in <2 weeks. A rapid postoperative decrease in retinal whitening (after 48 h) and artery reperfusion on fluorescein angiography was observed in three patients with branch RAO (fig 2), in another patient with branch RAO after vitreous haemorrhage clearing and in the patient with hemispheric artery occlusion (fig 3; 71.4%). In the remaining patient with branch RAO, a filling delay persisted in the affected arteriole despite embolus removal.
Figure 2 Top left: retina of a 67‐year‐old woman with inferotemporal artery occlusion of 22 h duration and visual acuity (VA) of counting fingers. Note the whitening of the retina, cherry red spot at the macular area and the embolus located at the optic disc (arrow). Top right: fluorescein angiography (40 s after intravenous infusion) before embolus removal. Bottom left: 48 h after surgery, retinal whitening markedly decreased and VA improved to 20/100. Two weeks later, VA had improved to 20/30. Bottom right: fluorescein angiography (28 s after intravenous infusion) 48 h after embolus removal. Perfusion of the affected area was re‐established; no staining was observed at the arteriolar wall.
Figure 3 Top left: retina of a 62‐year‐old man with an superohemispheric retinal artery occlusion of 12 h duration and visual acuity (VA) of hand movements. The embolus is located at the optic disc (arrow). Top right: fluorescein angiography shows absence of perfusion distal to the embolus (arrow). Bottom left: 48 h after surgery, retinal whitening decreased and VA improved to 20/200. The embolus has disappeared. The patient noticed a clear decrease in the superior scotoma. Bottom right: fluorescein angiography 48 h later shows reperfusion of the affected area and slight staining at the section site.
Postoperative best‐corrected visual acuity ranged from hand movements to 20/25 (median 20/40, IQR 20/30). Median postoperative macular thickness was 225 μm (IQR 101).
We found no cases of rubeosis iridis or neovascular glaucoma. Two patients (patients 1 and 3; table 1) developed cataracts and underwent phacoemulsification with intraocular lens placement in the capsular bag. Mean follow‐up was 12 months (range 7–21 months).
Discussion
Despite recent advances in vitreoretinal surgery, the visual prognosis for patients with RAO remains poor, with frequent permanent loss of vision or marked scotoma. Minimally invasive therapeutic options have been tried, including ocular massage,8 anterior chamber paracentesis,9 use of 95% oxygen and 5% carbon dioxide,10 isovolemic haemodilution,11 oral pentoxifylline, acetylsalicylate12 and hyperbaric oxygen therapy.13 Nevertheless, significant improvements in visual acuity have not been attained with one or a combination of these treatments.12 Local intra‐arterial fibrinolysis, mainly with recombinant tissue‐plasminogen activator14 and urokinase,15 has shown marginal visual benefits with potentially serious neurological complications. According to a meta‐analysis of the published data, evidence to justify fibrinolysis is insufficient.16
New procedures to treat central and branch RAO have been described, including translumenal neodymium:yttrium–aluminium–garnet (Nd:YAG) laser embolysis and surgical embolus removal. In two patients treated with Nd:YAG laser, Opremcak and Benner18 reported resolution of the emboli, immediate restoration of retinal blood flow and improved visual acuity. Reynard and Hanscom19 used Nd:YAG laser embolysis in one patient with central RAO and reported an improvement in visual acuity from counting fingers to 20/40. In our experience, Nd:YAG embolysis shows limited effectiveness and carries a high risk of vitreous haemorrhage.
Tang20 carried out surgical cannulation of the central retinal artery, with an improvement in visual acuity from counting fingers to 20/25 at 4 months. Mennel et al21 used radial optic neurotomy as a surgical option to treat combined cilioretinal artery and central retinal vein occlusion in a 64‐year‐old woman; visual acuity increased from 20/200 to 20/25.
Peyman and Gremillion17 treated one patient with embolic RAO of 60 h duration by surgical removal of one embolus and fragmentation with distal flow of 1 s. Visual acuity improved from counting fingers to 20/200.
We believed that the surgical approach proposed by Peyman would allow us direct mechanical access to the site of obstruction. Longitudinal section of the anterior arteriole wall is not a simple task because of the elasticity and displacement of the vessel during dissection, but improvements in vitreoretinal instruments, such as the 25‐guage microblade, facilitate the surgery. The initial incision had to be enlarged in all patients because the embolus was larger than the artery lumen. Vitreoretinal forceps were useful for expressing the embolus out of the artery when a section of the vessel alone did not suffice.
In one of the patients, reperfusion was not achieved even though the embolus was removed successfully. It is unclear whether the surgical aggression induced closure of the vessel or whether the embolus had produced prior, irreversible endothelial damage. Reperfusion was achieved by 48 h in four of the patients in whom the embolus was removed, and in three of these there was only slight staining of the vessel wall on fluorescein angiography. In the other patient, we had to wait for 2 weeks until the vitreous haemorrhage cleared, but perfusion of the arteriole was similar to the rest of the retina. One of the patients had good visual acuity (20/25), but had a complete visual field scotoma; 48 h after embolus removal, she noticed an improvement in the visual field defect.
The natural history of branch RAO is quite favourable: 80% of the eyes will eventually improve slowly after weeks or months to a visual acuity of 20/40, but many will have residual visual field defects. The short time interval to restoration of the visual field and visual acuity seen in this study suggests that surgical embolus removal may be beneficial. Experimental studies by Hayreh et al7 established that the retinal lesion in central RAO is irreversible after 4 h, but this interval may be longer in branch RAO because some degree of perfusion at the macular area may be supplied by the contralateral temporal artery.
The rapid improvement in our patients supports this theory. The potential risks of surgical embolus removal include retinal and vitreous haemorrhage, and the risks related to a pars plana vitrectomy. But the possibility of immediate vessel reperfusion and prompt visual acuity and visual field improvement needs to be weighed against these risks, in light of the permanent and often severe visual loss after RAO.
This is a pilot study, with the limitation of a series of only seven patients, but surgical embolus removal proved to be an effective method for reperfusing the occluded artery and improving the visual acuity and visual field in 71% of patients. Further experience with this technique and, perhaps, a randomised clinical trial are required to precisely define its role in retinal arteriolar occlusions.
Abbreviations
IQR - interquartile range
Nd:YAG - neodymium:yttrium–aluminium–garnet
RAO - retinal artery occlusion
Footnotes
Competing interests: None declared.
This study was presented in part at the Subspecialty Day at the Annual Meeting of the American Academy of Ophthalmology, Chicago, Illinois, USA, in October 2005.
References
- 1.Savino P J, Glaser J S, Cassady J. Retinal stroke: is the patient at risk? Arch Ophthalmol 1977951185–1189. [DOI] [PubMed] [Google Scholar]
- 2.Brown G C, Magargal L E. Central artery obstruction and visual acuity. Ophthalmology 19828914–19. [DOI] [PubMed] [Google Scholar]
- 3.Arruga J, Sanders M D. Ophthalmologic findings in 70 patients with evidence of retinal embolism. Ophthalmology 1982891336–1347. [DOI] [PubMed] [Google Scholar]
- 4.Ellis P P. Occlusion of the central retinal artery after retrobulbar corticosteroid injection. Am J Ophthalmol 197885352–356. [DOI] [PubMed] [Google Scholar]
- 5.Cogan D G, Wray S H. Vascular occlusions in the eye from cardiac myxomas. Am J Ophthalmol 197580396–403. [DOI] [PubMed] [Google Scholar]
- 6.Brown G C, Magargal L E, Simeone F E.et al Retinal arterial obstruction in children and young adults. Ophthalmology 19818818–25. [DOI] [PubMed] [Google Scholar]
- 7.Hayreh S S, Zimmerman M B, Kimura A.et al Central retinal artery occlusion. Retinal survival time. Exp Eye Res 200478723–736. [DOI] [PubMed] [Google Scholar]
- 8.Ffytche T J. A rationalization of treatment of central retinal artery occlusion. Trans Ophthalmol Soc UK 197494468–479. [PubMed] [Google Scholar]
- 9.Augsburger J J, Magargal L E. Visual prognosis following treatment of acute central retinal artery obstruction. Br J Ophthalmol 198064913–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frayser R, Hickham J B. Retinal vascular response to breathing increased carbon dioxide and oxygen concentrations. Invest Ophthalmol Vis Sci 19643427–431. [PubMed] [Google Scholar]
- 11.Rufer F, Schroder A, Winter R.et al Analysis of risk factors and comparison of heparin and hemodilution therapies for retinal artery occlusion. Ophthalmologe 2003100819–824. [DOI] [PubMed] [Google Scholar]
- 12.Mueller A J, Neubauer A S, Schaller U.et al Evaluation of minimally invasive therapies and rationale for a prospective randomized trial to evaluate selective intra‐arterial lysis for clinically complete central retinal artery occlusion. Arch Ophthalmol 20031211337–1381. [DOI] [PubMed] [Google Scholar]
- 13.Aisenbrey S, Krott R, Heller R.et al Hyperbaric oxygen therapy in retinal artery occlusion. Ophthalmologe 200097461–467. [DOI] [PubMed] [Google Scholar]
- 14.Richard G, Lerche R C, Knospe V.et al Treatment of retinal artery occlusion with local fibrinolysis using recombinant tissue plasminogen activator. Ophthalmology 1999106768–773. [DOI] [PubMed] [Google Scholar]
- 15.Butz B, Strotzer M, Manke C.et al Selective intraarterial fibrinolysis of acute central artery occlusion. Acta Radiol 200344680–684. [DOI] [PubMed] [Google Scholar]
- 16.Beatty S, Au K S. Local intra‐arterial fibrinolysis for acute occlusion of the central retinal artery: a meta‐analysis of the published data. Br J Ophthalmol 200084914–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peyman G A, Gremillion C M., Jr Surgical removal of a branch retinal artery embolus: a case report. Int Ophthalmol 199014295–298. [DOI] [PubMed] [Google Scholar]
- 18.Opremcak E M, Benner J D. Translumenal Nd:YAG laser embolysis for branch retinal artery occlusion. Retina 200222213–216. [DOI] [PubMed] [Google Scholar]
- 19.Reynard M, Hanscom T A. Neodymium:yttrium‐aluminum‐garnet laser arteriotomy with embolectomy for central retinal artery occlusion. Am J Ophthalmol 2004137196–198. [DOI] [PubMed] [Google Scholar]
- 20.Tang W M. Vitreous surgery for central retinal occlusion. Arch Ophthalmol 20001181586–1587. [PubMed] [Google Scholar]
- 21.Mennel S, Droutsas K, Meyer C H.et al Radial optic neurotomy in combined cilioretinal artery and central retinal vein occlusion. Br J Ophthalmol 200589642–643. [DOI] [PMC free article] [PubMed] [Google Scholar]