Abstract
Aim
To investigate the anatomical and functional results and the complications in eyes operated on using vitrectomy without scleral buckling for all forms of rhegmatogenous retinal detachment (RRD).
Methods
All cases of primary RRD at the University Hospital of Lund, Lund, Sweden, treated by one surgeon during a period of 3 years were retrospectively reviewed. In 131 (98%) of 134 consecutive cases, a final follow‐up record of 3–14 months was obtained, and these eyes were included in the study. The surgical protocol was tailored for each case and consisted of vitrectomy, laser photocoagulation and tamponade. Preoperative and intraoperative variables were analyses for risk for redetachment and postoperative proliferative vitreoretinopathy (PVR).
Results
Complete reattachment was achieved in 87% of cases (114/131) after one operation and in 95% cases after ⩾1 operation. A primary detachment of >1 quadrant was the only significant risk factor for redetachment (p<0.05). The most common cause of redetachment was progressive PVR. Significant risk and factors for PVR postoperatively were a poor preoperative visual acuity and a high number of laser effects during surgery (p<0.05). The visual acuity for the total number of eyes, macula‐off eyes, and pseudophakic as well as phakic eyes, improved significantly. The visual acuity for macula‐on eyes did not change significantly. Six patients developed ocular hypertension and another 6 an epiretinal membrane. Three patients reported a visual field defect. Increased lens opacification was seen in 64 of the 94 (68%) phakic eyes.
Conclusions
The tailored vitrectomy protocol is well suited to all types of RRD. Increased lens opacification in phakic eyes is common, but visual acuity is considerably improved in phakic as well as pseudophakic eyes. PVR development postoperatively is related to the extent of laser treatment, indicating that the protocol may be even further optimised in the future.
In most vitreoretinal surgical centres, the use of scleral buckling constitutes the mainstay of treatment for rhegmatogenous retinal detachment (RRD), either alone for routine cases or combined with vitrectomy for patients with more advanced forms of the disease.1,2,3,4 In recent years, however, reports on vitrectomy without scleral buckling for RRD have become more frequent.5,6,7,8,9,10,11,12,13,14 Vitrectomy offers the possibility of removing vitreoretinal traction, controlled drainage of subretinal fluid, and precise laser treatment. This so‐called internal approach has been found to be especially useful in eyes in which retinal breaks are large, posteriorly located or difficult to identify, and also in cases of media opacities. However, despite substantial technical development in the field, progress in RRD management in terms of both anatomical and functional results remains uncertain. This is shown by the fact that studies on scleral buckling as well as those on vitrectomy alone show a wide range of reattachment rates after one operation.1,2,3,4,5,6,7,8,9,10,11,12,13,14 The reasons behind these results may be diverging complex criteria for selection of patient material as well as the use of several surgical methods within each study.
Since 2001, FG has used a protocol for all patients with primary RRD in which vitrectomy and laser treatment are tailored for each patient according to the extent of detachment. The protocol has been developed to minimise the surgical trauma imposed by the vitrectomy and laser,15,16,17 and does not include the use of scleral buckle or encircling band. This article reports the anatomical and functional results, and the complications of non‐selected, consecutive patients treated during a 3‐year period.
Patients and Methods
Patients
A retrospective analysis was carried out of all cases (134 eyes of 134 patients) of primary rhegmatogenous retinal detachment (RRD) treated by FG at the University Hospital of Lund, Lund, Sweden, between August 2001 and July 2004. Retinal detachments after previous vitreoretinal surgery and secondary to trauma were not considered primary, and were thus not included. Data were obtained from patient records and from the surgical database at the Department of Ophthalmology, Lund University Hospital (FileMaker Pro, FileMaker, Santa Clara, California, USA). Two patients from other countries were lost to follow‐up, and one patient died 2 months after surgery, but in the remaining 131 (98%) cases, a record of final follow‐up examination was obtained 3–14 months (median 6 months, mean 7 months) postoperatively, and these patients were included in the study. Eyes in which gas had been used as tamponade (see below) and with no postoperative complications were followed up for 3–6 months. Eyes in which silicon oil had been used, as well as eyes with complications (redetachment, cataract, epiretinal membrane (ERM), raised intraocular pressure or visual field defect), were followed up for longer. We wanted an evaluation of detachment surgery only, and the time of final examination was thus chosen before additional procedures such as phaco/intraocular lens (IOL) or ERM peeling.
Background data for several preoperative variables were obtained, and included age, sex, other eye diseases, duration of symptoms, preoperative visual acuity, lens status and presence of vitreous haemorrhage (table 1). Detachments were characterised in terms of extent, location, number and location of breaks, and macular involvement. The grade of PVR was determined according to the Retina Society classification.18
Table 1 Preoperative background data of patients (n = 131).
| Variable | Data | Mean (SD) | Frequency | % |
|---|---|---|---|---|
| Age (years) | All | 62.8 (12.6) | ||
| Sex | Male | 76 | 58 | |
| Female | 55 | 42 | ||
| Concomitant eye diseases | Amblyopia | 6 | 5 | |
| Retinoschisis | 1 | 1 | ||
| Epiretinal membrane | 1 | 1 | ||
| Glaucoma | 4 | 3 | ||
| Age‐related macular degeneration | 12 | 9 | ||
| High myopia | 7 | 6 | ||
| Duration of symptoms (days) | All | 11.8 (17.4) | ||
| Preoperative VA (logMAR) | All | 1.15 (1.14) | ||
| Preoperative IOP (mm Hg) | n = 117 | 13.9 (4.1) | ||
| No data, n = 14 | ||||
| Lens status | Phakic | 94 | 72 | |
| Pseudophakic | 37 | 28 | ||
| Aphakic | 0 | 0 | ||
| Vitreous haemorrhage | Yes | 13 | 10 | |
| No | 118 | 90 | ||
| Extent of detachment (quadrants) | 1 | 32 | 24 | |
| 2–4 | 99 | 76 | ||
| Vertical location of detachment | Superior quadrants | 68 | 52 | |
| Inferior quadrants | 37 | 28 | ||
| Equal distribution | 26 | 20 | ||
| Retinal breaks | 1 | 71 | 54 | |
| ⩾2 | 50 | 38 | ||
| Not found | 10 | 8 | ||
| Inferior breaks (4–8 o'clock) | Yes | 30 | 23 | |
| No | 91 | 69 | ||
| Macular status | On | 49 | 37 | |
| Off | 80 | 61 | ||
| No data | 2 | 2 | ||
| Preoperative PVR grades | 0 | 13 | 10 | |
| A | 103 | 79 | ||
| B | 6 | 4 | ||
| C1–C3 | 9 | 7 |
IOP, intraocular pressure; logMAR, logarithm of the minimum angle of resolution; PVR, proliferative vitreoretinopathy; VA, visual acuity.
Items are presented as categorical data with absolute and relative frequencies or as numerical data with mean (SD).
Surgery
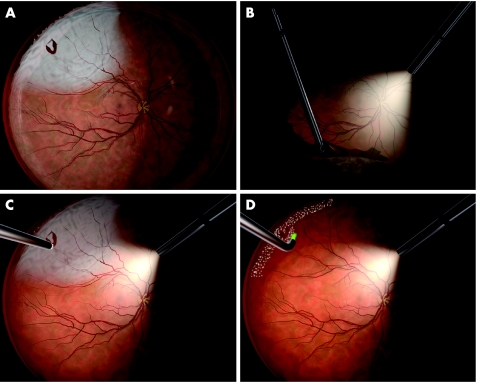
FG carried out all primary operations. No encircling band or buckle was used. A three‐port vitrectomy using a 110D BIOM lens was carried out with the Alcon Accurus probe (n = 32) or Alcon Innovit probe (n = 99; Alcon Laboratories, Forth Worth, Texas, USA). Balanced salt solution (BSS, Alcon Laboratories) was used for infusion in pseudophakic and BSS as well as phakic eyes. By using scleral indentation, all vitreous in the quadrants corresponding to the detachment was removed. The inferior peripheral vitreous was also removed using this method (fig 1B). The peripheral vitreous corresponding to the remaining attached retina was retained. The subretinal fluid was drained through existing breaks in 100 (76%) eyes or through a retinotomy in 31 (24%) eyes using air–fluid exchange (fig 1C). The decision to carry out a drainage retinotomy was made when no retinal rupture was identified (n = 10), or when a small break was detected in the far periphery (n = 21). Perfluorooctane (Okta‐line, Bausch & Lomb Surgical, Claremont, California, USA) was used in 4 eyes. Epiretinal proliferative vitreoretinopathy (PVR) membranes were peeled in 6 eyes, subretinal PVR membranes extracted in 2 eyes and a limited peripheral retinectomy was carried out in 1 eye. Endolaser retinopexy was carried out in all eyes (OcuLight GL, IRIDEX, Mountainview, California, USA) with treatment of retinal breaks, as well as a peripheral scatter corresponding to the extension of the RD (fig 1D). Any attached parts of the retina showing degenerative changes were also treated, but remaining parts were left untreated. A total of 134–840 effects (mean (standard deviation(SD)) 423 (147)) were used. Sulphur hexafluoride (SF6) 10–25% was used in most cases as tamponade (n = 101). Hexafluoroethane (C2F6) 12–20% (n = 3) or perfluoropropane (C3F8) 14–20% (n = 13) was used in eyes with detachments of long duration with PVR grade 0–A. Silicone oil 1000–5000 cstk (n = 14) was used in eyes with more severe PVR. In all, 114 (87%) eyes were operated under local anaesthesia (8 retrobulbar and 106 peribulbar), and the remaining 17 (13%) surgeries were carried out under general anaesthesia. In one case, the procedure was combined with the removal of an anterior chamber intraocular lens, and this operation lasted 122 min. In the remaining 130 cases, the operation time ranged from 27 to 97 min (mean (SD) 54.4 (13.4) min).
Figure 1 The tailored vitrectomy protocol for rhegmatogenous retinal detachment. This eye shows a single break and surrounding detachment in the superior temporal quadrant (A). By using scleral indentation, all vitreous in the quadrant corresponding to the detachment is removed, along with the inferior peripheral vitreous (B). Drainage of the subretinal fluid is made through the existing break (C) and endolaser retinopexy is used to seal the break and to treat the peripheral retina corresponding to the extension of the detachment (D).
All patients were admitted to the Department of Ophthalmology, Lund University Hospital, Lund, Sweden, and they stayed for 1–8 days. Patients were asked to maintain a face‐down position as much possible for 7 days after the operation.
Statistical analysis
Fourteen preoperative and intraoperative variables were analysed for risk of redetachment, and eight variables were analysed for risk of postoperative PVR. Categorical variables were analysed using Fisher's exact test, and numerical variables with the Mann–Whitney U test. Visual acuity was measured on decimal Snellen charts, but was converted to logarithm of the minimum angle of resolution (logMAR) units for statistical analyses. Hand motions and light perception were assigned the arbitrary Snellen decimal values of 0.001 (logMAR 3.0) and 0.0005 (logMAR 3.3), respectively. Preoperative and postoperative visual acuity were compared using the two‐tailed Wilcoxon matched pairs signed ranks test. Data were analysed using GraphPad InStat (GraphPad Software, San Diego, California, USA). Significance was defined at p<0.05 using the above‐mentioned tests.
Results
Retinal reattachment
The retina was completely attached in 114 of the 131 (87%) eyes after one operation, and in 124 (95%) eyes after 2–3 operations. Redetachments occurred after 12–78 days in 17 eyes, and were caused by progressive PVR in 10 eyes, and by new breaks in 4 eyes. In 1 eye the primary retinal break was not sufficiently closed, and in 2 eyes no record of redetachment cause was obtained. A further analysis of the redetached eyes showed that an initial detachment of ⩾2 quadrants (16 of 17 eyes) was a significant risk factor for redetachment (table 2). None of the remaining 13 variables were found to be a significant risk factor for redetachment. Variables of borderline significance included use of retinotomy and surgery during the first 18 months of the study period. None of the 15 eyes with preoperative PVR grade B–C3 redetached. In the 10 eyes that developed postoperative PVR, significantly more laser treatment had been administered than in eyes without PVR, and these eyes also had a poorer preoperative logMAR visual acuity (table 3).
Table 2 Analysis of risk factors for redetachment.
| Variable | Data | Mean (SD) | Frequency of redetachment/total number | Percentage | p Value |
|---|---|---|---|---|---|
| Duration of symptoms (days)* | Redetached | 11.3 (15.8) | 0.36 | ||
| Attached | 11.8 (17.7) | ||||
| Preoperative VA (logMAR)* | Redetached | 1.47 (1.20) | 0.15 | ||
| Attached | 1.10 (1.13) | ||||
| Lens status | Phakic | 13/94 | 14 | 0.77 | |
| Pseudophakic | 4/37 | 11 | |||
| Vitreous haemorrhage | No | 16/118 | 14 | 0.47 | |
| Yes | 1/13 | 8 | |||
| Extent of detachment (quadrants) | 1 | 1/32 | 3 | 0.045 | |
| 2–4 | 16/99 | 16 | |||
| Vertical location of detachment | Inferior quadrants | 4/37 | 11 | 0.39 | |
| Superior quadrants | 5/68 | 7 | |||
| Retinal breaks, number | 1 | 7/71 | 10 | ||
| 2–4 | 8/50 | 16 | 0.29 | ||
| Not found | 2/10 | 20 | 0.38 | ||
| Inferior breaks (4–8 o'clock) | Yes | 6/30 | 20 | 0.13 | |
| No | 9/91 | 10 | |||
| Macular status | Off | 12/80 | 15 | 0.435 | |
| On | 5/49 | 10 | |||
| Preoperative PVR grade | 0–A | 17/116 | 15 | 0.11 | |
| B–C3 | 0/15 | 0 | |||
| Retinotomy | Yes | 7/31 | 23 | 0.070 | |
| No | 10/100 | 10 | |||
| Intraoperative tamponade | SF6 | 12/101 | 12 | 0.13 | |
| C3F8 or C2F6 | 4/16 | 25 | |||
| Silicone oil | 1/14 | 7 | |||
| Laser effects* | Redetached | 454 (156) | 0.16 | ||
| Attached | 418 (147) | ||||
| Period of surgery | First 18 months | 12/68 | 18 | 0.081 | |
| Last 18 months | 5/63 | 8 |
C2F6, hexafluoroethane; C3F8, perfluoropropane; logMAR, logarithm of the minimum angle of resolution; PVR, proliferative vitreo retinopathy; SF6, sulphur hexafluoride; VA, visual acuity.
Eyes with redetachment (n = 17) compared with eyes where the retina was completely attached (n = 114). Data were analysed as categorical variables using Fisher's exact test or as numerical variables (*) using the Mann–Whitney U test.
Table 3 Analysis of risk factors for postoperative proliferative vitreoretinopathy.
| Variable | Data | Mean (SD) | Frequency of postoperative PVR/total number | % | p Value |
|---|---|---|---|---|---|
| Preoperative VA (logMAR)* | Postoperative PVR | 1.74 (1.04) | 0.023 | ||
| No postoperative PVR | 1.10 (1.14) | ||||
| Extent of detachment (quadrants) | 1 | 1/32 | 3 | 0.55 | |
| 2–4 | 9/99 | 9 | |||
| Retinal breaks, number | 1 | 5/71 | 7 | ||
| 2–4 | 4/50 | 8 | |||
| Macular status | Off | 9/80 | 11 | 0.052 | |
| On | 1/49 | 2 | |||
| Retinotomy | Yes | 4/31 | 13 | 0.19 | |
| No | 6/100 | 6 | |||
| Intraoperative tamponade | SF6 | 6/101 | 6 | 0.11 | |
| C3F8 or C2F6 | 3/16 | 19 | |||
| Silicone oil | 1/14 | 7 | |||
| Laser effects* | Postoperative PVR | 530 (145) | 0.006 | ||
| No postoperative PVR | 414 (145) | ||||
| Period of surgery | First 18 months | 8/68 | 12 | 0.062 | |
| Last 18 months | 2/63 | 3 |
C2F6, hexafluoroethane; C3F8, perfluoropropane; logMAR, logarithm of the minimum angle of resolution; PVR, proliferative vitreoretinopathy; SF6, sulphur hexafluoride; VA, visual acuity.
Eyes with postoperative PVR (n = 10) compared with eyes without PVR (n = 121). Data were analysed as categorical variables using Fisher's exact test or as numerical variables (*) using the Mann–Whitney U test.
Of the 17 patients with a redetachment after the first operation, 1 declined further surgery. In the remaining eyes, subsequent operations, carried out by 1 of 4 consultants including FG, involved a more extensive vitrectomy in 15 eyes and an external fluid–gas exchange combined with laser photocoagulation in 1 eye. No encircling band was placed during reoperations.
At final examination, 8 eyes had remaining silicone oil from the primary operation, most of which later underwent combined oil evacuation and cataract surgery. In 7 of the 17 (5% of the total 131 eyes) redetached eyes, detachment persisted despite using silicone oil at reoperation. In 4 of these eyes the central part of the retina was attached, but a limited peripheral detachment was evident. The remaining 3 (2%) eyes showed a detachment involving the major part of the retina.
Complications
One patient displayed a vitreous haemorrhage 6 days after surgery, which completely resolved in a month. In all, 8 (6%) patients required additional treatment for raised intraocular pressure postoperatively. Two of these patients had been diagnosed with ocular hypertension preoperatively. In 5 eyes, the intraocular pressure returned to normal in a few weeks, whereas 3 patients still required topical medication at final follow‐up. Increased lens opacification was seen in 64 of the 94 (68%) phakic eyes. Forty eight (81%) of the 59 patients with a clear lens preoperatively developed this complication. Six (5%) patients developed an ERM. Three of these eyes displayed an ERM where peeling was warranted. Three (2%) patients reported a visual field defect in the operated eye. In these cases, all of which included the right eye, Goldmann perimetry showed defects in the lower temporal visual field.
Visual acuity
Table 4 shows preoperative and postoperative visual acuity for all patients, and for subgroups divided according to lens and macular status. To summarise, the visual acuity for the total number of eyes, macula‐off eyes and pseudophakic as well as phakic eyes improved significantly after surgery. The visual acuity for macula‐on eyes did not change significantly. In all, 115 (89%) eyes had a visual acuity of ⩾0.1 and 83 (63%) eyes had a visual acuity of ⩾0.4 at final postoperative examination.
Table 4 Preoperative and postoperative visual acuity of patients.
| Variable | Data | n | Preoperative VA | Postoperative VA | p Value | ||
|---|---|---|---|---|---|---|---|
| logMAR (mean (SD)) | Snellen (median) | logMAR (mean (SD)) | Snellen (median) | ||||
| All | 131 | 1.15 (1.14) | 0.2 | 0.59 (0.70) | 0.5 | <0.001 | |
| Lens status | Phakic | 94 | 1.04 (1.10) | 0.2 | 0.65 (0.72) | 0.4 | 0.003 |
| Pseudophakic | 37 | 1.41 (1.21) | 0.1 | 0.44 (0.64) | 0.6 | <0.001 | |
| Macular status | On | 49 | 0.25 (0.41) | 0.8 | 0.32 (0.53) | 0.7 | 0.075 |
| Off | 80 | 1.68 (1.10) | 0.04 | 0.75 (0.76) | 0.4 | <0.001 | |
| No data | 2 | ||||||
logMAR, logarithm of the minimum angle of resolution; VA, visual acuity.
Statistical comparison of preoperative and postoperative logMAR VA was made using the two‐tailed Wilcoxon's matched pairs signed ranks test.
Discussion
As with most diseases, the clinical presentation of eyes with RRD varies to a certain degree. Traditionally, the number and location of breaks, extent of detachment, grade of PVR and presence of vitreous haemorrhage are among the many factors considered when a surgical strategy is planned. As a consequence, a multitude of procedures exist, with various components derived from scientific data that are often limited. The seemingly endless discussion of scleral buckling versus vitrectomy for RRD illustrates well that the choice of operation is dependent more on the personal preference of the surgeon than on the actual presentation of RRD.19,20,21,22,23,24,25,26 Even well‐intended, ambitious prospective studies are severely hampered by the many surgical options, making scientific evaluation difficult. In this setting, a more standardised approach to RRD management may be advantageous in limiting preoperative choices, thereby enabling a more strict analysis of obtained results. In the surgical protocol presented here, surgery was planned individually on the basis of the extent and duration of detachment and grade of PVR, but surgical options were limited to the extent of vitreous removal, the extent of laser photocoagulation and the choice of tamponade. Although the extent of detachment at presentation was considerably associated with redetachment, other traditional variables such as high grade of PVR, long duration of detachment, poor visual acuity, presence of vitreous haemorrhage, inferior breaks and multiple retinal breaks were not associated with an increased risk.13,27,28 We thus conclude that our surgical protocol is suitable for all types of primary RRD, regardless of clinical presentation, and that both anatomical and functional results are excellent.
Owing to the aforementioned diversity of existing surgical procedures and the tendency to include selected instead of consecutive cases, it is difficult to compare our results with those from previous reports on vitrectomy without buckling for RRD.5,6,7,8,9,10,11,12,13,14 Several studies on vitrectomy alone for RRD include results in which the vitrectomy in some of the eyes has been combined with a buckling procedure.10,11,12,13,14 The number of studies in which vitrectomy alone has been used on all patients is few, and these studies often exclude several forms of the disease—for example, eyes with severe PVR.6,7,9 Other studies exclude patients depending on their lens status.7,8,9 To our knowledge, our study is the first attempt to report vitrectomy alone as a treatment for consecutive cases of patients with all forms of RRD.
The rationale behind the tailored vitrectomy protocol relies on both mechanical and biochemical factors. The common strategy for any surgical treatment of RRD is to close all retinal breaks by release of vitreal traction, use of retinopexy and, in most protocols, also tamponade. Surgical intervention, however, incurs the risk of complications. One of the most devastating consequences of surgical treatment of RRD, either internal or external, is the induction of progressive PVR, which in several studies has been identified as the single most important factor for redetachment.2,8,29 The cause of postoperative PVR is probably multifactorial, and is dependent on the presence of two preconditions—inflammation and responsive cells.30 The logical goal of a vitrectomy procedure in RRD would be to remove such cells and also their substrates of attachment (vitreous collagen) without causing an increased inflammatory response. This, however, is a perilous task, which is evident by the fact that the actual use of vitrectomy as well as laser photocoagulation has been identified as a risk factor for PVR development.15,16 In previous studies, the degree of vitreous removal ranges from a core vitrectomy followed by removal of the vitreous adherent to breaks,5 to a complete 360° shaving of the vitreous base.7,9 Similarly, the protocol for retinopexy varies from local5,6,8,9 to 360° treatment,7 with either cryo or laser photocoagulation. The surgical protocol presented here with limited vitrectomy and laser retinopexy can be seen as a compromise, aimed at the removal of vitreal traction and responsive cells, with a minimal induction of inflammation. However, despite our efforts to restrict surgical intervention, PVR was still the most common cause of redetachment in our series of patients. Of the 10 eyes with postoperative PVR, 9 had a primary detachment of ⩾2 quadrants and, consequently, vitreous removal and laser photocoagulation was comparatively extensive. Interestingly, the extent of the detachment and multiple breaks was not a major risk factor for PVR, whereas a high number of laser spots were highly relevant. Additionally, 9 of the 10 eyes with PVR had a detachment involving the macula, and poor preoperative visual acuity was also found to be a major risk factor for postoperative PVR. These results indicate that when the potentially inflammation‐inducing intervention of extensive endolaser retinopexy is imposed on a detached neuroretina already under ischaemic stress, the response is inevitably PVR formation. Whether an even more limited surgical protocol—that is, with retinopexy of breaks and retinal degenerative areas only—can help to reduce postoperative PVR will certainly be investigated in the future.
The most common complication of vitrectomy is cataract formation in phakic eyes. In accordance with previous studies on vitrectomy for RRD as well as for macular holes, and despite our observation of an increased lens opacification in most phakic eyes,6,31 visual acuity was greatly improved postoperatively in eyes with phakia and those with pseudophakia. However, the follow‐up time of our study was comparatively short, and many of the phakic eyes will most probably at some point require cataract removal. Even if cataract surgery has developed considerably in the past decade, cataract formation after vitrectomy remains a major inconvenience, especially for young patients still retaining accomodation.32 One way of handling the problem is to combine the vitrectomy with cataract surgery, a procedure that is now becoming more common.11,13 Our protocol for RRD management is based on the strategy that surgical trauma to the ocular tissues should be minimised, and a perhaps more attractive development is the use of air as tamponade for patients with pseudophakic RRD with inferior ruptures.9 If these results can be extrapolated to patients with phakia, the relatively short duration of the air tamponade may reduce postoperative cataract formation.32
Abbreviations
ERM - epiretinal membrane
IOL - intraocular lens
logMAR - logarithm of the minimum angle of resolution
PVR - proliferative vitreo retinopathy
RRD - rhegmatogenous retinal detachment
Footnotes
Funding: This study was supported by The Faculty of Medicine, University of Lund, The Swedish Research Council, The Princess Margaretas Foundation for Blind Children, The Maggie Stephen foundation and The R&D Centre Kronoberg County Council.
Competing interests: None declared.
References
- 1.Tani P, Robertson D M, Langworthy A. Prognosis for central vision and anatomic reattachment in rhegmatogenous retinal detachment with macula detached. Am J Ophthalmol 198192611–620. [DOI] [PubMed] [Google Scholar]
- 2.La Heij E C, Derhaag P F J M, Hendrikse F. Results of scleral buckling operations in primary rhegmatogenous retinal detachment. Doc Ophthalmol 200010017–25. [DOI] [PubMed] [Google Scholar]
- 3.Sharma T, Challa J K, Ravishankar K V.et al Scleral buckling for retinal detachment. Retina 199414338–343. [DOI] [PubMed] [Google Scholar]
- 4.Devenyi R G, de Carvalho Nakamura H. Combined scleral buckle and pars plana vitrectomy as a primary procedure for pseudophakic retinal detachments. Ophthalmic Surg Lasers 199930615–618. [PubMed] [Google Scholar]
- 5.Escoffery R F, Olk R J, Grand M G.et al Vitrectomy without scleral buckling for primary rhegmatogenous retinal detachment. Am J Ophthalmol 198599275–281. [DOI] [PubMed] [Google Scholar]
- 6.Heimann H, Bornfeld N, Friedrichs W.et al Primary vitrectomy without scleral buckling for rhegmatogenous retinal detachment. Graefe's Arch Clin Exp Ophthalmol 1996234561–568. [DOI] [PubMed] [Google Scholar]
- 7.Campo R V, Sipperly J O, Sneed S R.et al Pars plana vitrectomy without scleral buckle for pseudophakic retinal detachments. Ophthalmology 19991061811–1816. [DOI] [PubMed] [Google Scholar]
- 8.Speicher M A, Fu A D, Martin J P.et al Primary vitrectomy alone for repair of retinal detachments following cataract surgery. Retina 200020459–464. [DOI] [PubMed] [Google Scholar]
- 9.Martinez‐Castillo V, Boixadera A, Verdugo A.et al Pars plana vitrectomy alone for the management of inferior breaks in pseudophakic retinal detachment without facedown position. Ophthalmology 20051121222–1226. [DOI] [PubMed] [Google Scholar]
- 10.Hakin K N, Lavin M J, Leaver P K. Primary vitrectomy for rhegmatogenous retinal detachment. Graefe's Arch Clin Exp Ophthalmol 1993231344–346. [DOI] [PubMed] [Google Scholar]
- 11.Oshima Y, Emi K, Motokura M.et al Survey of surgical indications and results of primary pars plana vitrectomy for rhegmatogenous retinal detachment. Jpn J Ophthalmol 199943120–126. [DOI] [PubMed] [Google Scholar]
- 12.Newman D K, Burton R L. Primary vitrectomy for pseudophakic and aphakic retinal detachments. Eye 199913635–639. [DOI] [PubMed] [Google Scholar]
- 13.Heimann H, Zou X, Jandeck C.et al Primary vitrectomy for rhegmatogenous retinal detachment: an analysis of 512 cases. Graefe's Arch Clin Exp Ophthalmol 200624469–78. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt J C, Rodrigues E B, Hoerle S.et al Primary vitrectomy in complicated rhegmatogenous retinal detachment—a survey of 205 eyes. Ophthalmologica 2003217387–392. [DOI] [PubMed] [Google Scholar]
- 15.Cowley M, Conway B P, Campochiaro P A.et al Clinical risk factors for proliferatve vitreoretinopathy. Arch Ophthalmol 19891071147–1151. [DOI] [PubMed] [Google Scholar]
- 16.Algvere P, Hallnäs K, Dafgård E.et al Panretinal photocoagulation aggravates experimental proliferative vitreoretinopathy. Graefe's Arch Clin Exp Ophthalmol 1990228461–466. [DOI] [PubMed] [Google Scholar]
- 17.Yoshida A, Ishiguro S, Tamai M. Express of glial fibrillary acidic protein in rabbit Müller cells after lensectomy‐vitrectomy. Invest Ophthalmol Vis Sci 1993343154–3160. [PubMed] [Google Scholar]
- 18.The Retina Society Terminology Committee The classification of retinal detachment with proliferative vitreoretinopathy. Ophthalmology 198390121–125. [DOI] [PubMed] [Google Scholar]
- 19.Woon W H, Burdon M A, Green W T.et al Comparison of pars plana vitrectomy and scleral buckling for uncomplicated rhegmatogenous retinal detachment. Curr Opin Ophthalmol 1995676–79. [DOI] [PubMed] [Google Scholar]
- 20.Wolfensberger T J. Foveal reattachment after macula‐off retinal detachment occurs faster after vitrectomy than after buckle surgery. Ophthalmology 20041111340–1343. [DOI] [PubMed] [Google Scholar]
- 21.Oshima Y, Yamanishi S, Sawa M.et al Two‐year follow‐up study comparing primary vitrectomy with scleral buckling for macula‐off rhegmatogenous retinal detachment. Jpn J Ophthalmol 200044538–549. [DOI] [PubMed] [Google Scholar]
- 22.Heimann H, Hellmich M, Bornfeld N.et al Scleral buckling versus primary vitrectomy in rhegmatogenous retinal detachment (SPR Study): design issues and implications. SPR Study Report No 1. Graefe's Arch Clin Exp Ophthalmol 2001239567–574. [DOI] [PubMed] [Google Scholar]
- 23.Ahmadieh H, Moradian S, Faghihi H.et al Pseudophakic and Aphakic Retinal Detachment (PARD) Study Group. Anatomic and visual outcomes of scleral buckling versus primary vitrectomy in pseudophakic and aphakic retinal detachment: six‐month follow‐up results of a single operation—report no, 1. Ophthalmology 20051121421–1429. [DOI] [PubMed] [Google Scholar]
- 24.Miki D, Hida T, Hotta K.et al Comparison of scleral buckling and vitrectomy for retinal detachment resulting from flap tears in superior quadrants. Jpn J Ophthalmol 200145187–191. [DOI] [PubMed] [Google Scholar]
- 25.Salicone A, Smiddy W E, Venkatraman A.et al Management of retinal detachment when no break is found. Ophthalmology 2006113398–403. [DOI] [PubMed] [Google Scholar]
- 26.Brazitikos P D, Androudi S, Christen W G.et al Primary pars plana vitrectomy versus scleral buckle surgery for the treatment of pseudophakic retinal detachment: a randomized clinical trial. Retina 200525957–964. [DOI] [PubMed] [Google Scholar]
- 27.Greven C M, Sanders R J, Brown G C.et al Pseudophakic retinal detachments. Anatomic and visual results. Ophthalmology 199299257–262. [DOI] [PubMed] [Google Scholar]
- 28.Grizzard W S, Hilton G F, Hammer M E.et al A multivariate analysis of anatomic success of retinal detachments treated with scleral buckling. Graefe's Arch Clin Exp Ophtahalmol 19942321–7. [DOI] [PubMed] [Google Scholar]
- 29.Rachal W F, Burton T C. Changing concepts of failures after retinal detachment surgery. Arch Ophthalmol 197997480–483. [DOI] [PubMed] [Google Scholar]
- 30.Kirchhof B. Strategies to influence PVR development. Graefe's Arch Clin Exp Ophtahalmol 2004242699–703. [DOI] [PubMed] [Google Scholar]
- 31.Livingstone B I, Bourke Fraco R D. Retrospective study of macular holes treated with pars plana vitrectomy. Aust N Z J Ophthalmol 199927331–341. [DOI] [PubMed] [Google Scholar]
- 32.Blodi B A, Paluska S A. Cataract after vitrectomy in young patients. Ophthalmology 19971041092–1095. [DOI] [PubMed] [Google Scholar]