Abstract
Aims
To show the refractive outcomes, accuracy of intraocular lens power selection, and visual outcomes and complications in infants undergoing cataract surgery.
Methods
The refraction (spherical equivalent) of 14 operated eyes in 8 children aged <1 year was plotted over time. Preoperative and final recorded visual acuities were assessed.
Results
The median follow‐up was 37.25 months. The median initial postoperative refraction was +6.75 dioptres.
Conclusions
Refractive outcomes for each eye were not entirely predictable and were variable between infants. However, there was a consistent pattern in each infant who underwent bilateral surgery, with both eyes following a similar pattern of refractive change with time: a decreasing myopic shift was seen in 8 eyes, possibly demonstrating emmetropisation. The two unilateral cases appeared to show a linear myopic shift. 4 eyes in 2 patients did not follow a myopic shift curve and one of these patients showed an early trend towards increased hyperopia. Definite causes for this erratic refractive change were not identified. A postoperative refraction >4.5 dioptres avoided early onset myopia.
The range of difference between postoperative and predicted refraction using SRK‐T was −2.85 to 2.97 dioptres.
Most of the visual results are encouraging compared with historical data in older children.
Refractive changes after congenital cataract surgery in infants could affect their visual development. The choice of intraocular lens (IOL) power is therefore important. Children's eyes normally undergo a small myopic shift as they grow: from +0.4 dioptres (D) at birth to −0.5 D in adults.1 This is despite large axial length changes: from 16.8 mm at birth to 23.6 mm in adult life.2 A reduction in lens power during this time accounts for the relatively small myopic shift. In pseudophakia and aphakia, this limitation of myopic shift cannot occur. In one paper, pseudophakic eyes showed less myopic shift than age‐matched aphakic eyes. However, these children had on average their cataract surgery at about 7 years.3 Mathematical estimates of the postoperative refractive change (PORC) that is likely in pseudophakic children exist,4 and data are available for PORC in children of at least 3 years.5 Some reports have shown that PORC in pseudophakic children is greater with decreasing age at the time of surgery.6,7
Many surgeons now implant IOLs in infants during cataract surgery. This retrospective consecutive case‐series outcome analysis aims to give early data on the refractive and visual outcomes in such infants. Other aims are to determine if IOL power determination needs modification and whether refractive factors have a disproportionate effect on visual results, as well as defining the frequency of further surgery required and complication rates.
Methods
Eyes of infants (<1 year of age) undergoing congenital cataract surgery by a surgeon (AGQ) aiming for IOL implantation in the bag and pars plana or plicata anterior vitrectomy, and posterior capsulectomy from June 2000 to September 2003 were included. Bilateral cases were categorised as Group A and unilateral cases as Group B. Spherical equivalent refractions (in D sphere) at postoperative visits were plotted over time. Dilated retinoscopy was carried out by an optometrist with extensive paediatric experience or, occasionally, by the surgeon. Biometry was performed under general anaesthesia using a Tomey® A scan ultrasound device (Tomey Coporation, Nagoya, Japan; Bio and Pachymeter AL‐1000), which required direct corneal contact to measure axial length; keratometry values were obtained using a Nidek hand‐held autokeratometer (Nidek USA, California, USA) or Nikon Retinomax K plus autorefractor (Nikon Corporation, Tokyo, Japan). The postoperative aim was for about +6.00 D sphere hyperopia (using SRK‐T) in seven eyes (table 1). The intraocular pressure was measured almost exclusively with a tonopen, most often with the infants awake; some measurements were made at induction under general anaesthesia during examinations under anaesthetics (EUAs).
Table 1 Axial length at surgery, refractive target and postoperative refraction.
| Patient | Eye | Axial length (mm) | Target post‐op refraction (D) | Implant power and type | Measured first post‐op refraction | Refraction at 3 years or latest (L) |
|---|---|---|---|---|---|---|
| 1 | Left | 19.78 | 6.11 | 24 D Pharmacia | 4.00 | −1.78 |
| Right | 19.68 | 6.00 | 24 D Pharmacia | 4.50 | 0.04 | |
| 2 | Right | 17.79 | 8.88 | 28 D Pharmacia | 8.00 | 4.35 |
| Left | 17.8 | 9.53 | 27 D Pharmacia | 10.00 | 2.35 | |
| 3 | Left | 18.62 | 5.90 | 28 D Acrysof | 8.75 | 0.25 |
| Right | 20.76 | 4.60 | 24 D Acrysof | 5.50 | 1.56 | |
| 4 | Right | 18.6 | 7.30 | 30 D Acrysof | 5.00 | 4.67 |
| Left | 18.81 | 6.28 | 30 D Acrysof | 4.25 | 5.85 | |
| 5 | Right | 17.02 | 9.56 | 30 D Acrysof | 8.25 | 6 (L) |
| Left | 16.84 | 9.92 | 30 D Acrysof | 9.50 | 7 (L) | |
| 6 | Right | 17.22 | 6.88 | 30 D Acrysof | 8.25 | −0.63 (L) |
| Left | 18.66 | 6.41 | 30 D Acrysof | 8.00 | −0.13 (L) | |
| 7 | Right | 18.84 | 6.35 | 29 D Acrysof | 3.38 | −2 (L) |
| No op | — | — | — | — | — | |
| 8 | Left | 21.98 | 1.84 | 23 D Acrysof | 2.00 | −1 (L) |
| No op |
op, operation; Pharmacia intraocular lens (IOLs) were 811C heparin‐coated; Acrysof IOLs were MA60; median first postoperative (post‐op) refraction at 14 days post‐op (range 1–60 days); median first postop refraction = 6.75 D sphere (range 2–10); median difference (target post‐op refraction—measured first post‐op refraction) = 0.65 D sphere; range (−2.85 to 2.97 D sphere); median refraction at 3 years (estimated from graphs, n = 8 eyes of 4 patients) post‐op = 1.96 D sphere.
All infants were prescribed spectacles (overcorrected by +2‐D sphere initially and reduced as clinically appropriate) after the operation. Bifocal spectacles were introduced at the appropriate time. Linear, logarithmic, exponential and power functions were assessed for their appropriateness in fitting to the data in each case using R2 values.
Visual acuity data were also recorded. Associated ocular and systemic pathologies were recorded during a systematic notes review (table 2), as was any requirement for further ocular surgery.
Table 2 Visual acuities, age at surgery, follow‐up length and associations.
| Patient | Eye | VA pre‐op | Age at op | Final VA | Follow‐up (months) | Associations |
|---|---|---|---|---|---|---|
| 1 | Left | 6/620 | 30/52 | 2/30 CrK | 49.25 | Nystagmus pre‐op |
| Right | 33/52 | 3/24 CrK | 48.50 | |||
| 2 | Right | No fixation | 4/52 | 6/6 Sn | 58.25 | |
| Left | No fixation | 6/52 | 6/9+2 Sn | 57.75 | ||
| 3 | Left | 6/500 | 42/52 | 1/30 CrK | 37.25 | Nystagmus pre‐op |
| Right | No fixation | 40/52 | 3/4.8 CrK | 37.75 | ||
| 4 | Right | Fix and follow | 14/52 | 3/3.8 CrK | 38.50 | |
| Left | Fix and follow | 19/52 | 3/6 CrK | 37.25 | ||
| 5 | Right | No fixation | 15/52 | 3/6 CCa | 25.50 | Nystagmus pre‐op |
| Left | No fixation | 11/52 | 3/9 CCa | 26.50 | Microphthalmos BE | |
| 6 | Right | 6/1000 | 9/52 | 3/9 SiK | 25.50 | Microphthalmos |
| Left | 12/52 | 3/9 SiK | 24.75 | |||
| 7 | Right | 6/620 | 19/52 | 3/4.8 CrK | 32.50 | Divergent squint |
| No op | >6/60 | 3/3.8 CrK | ||||
| 8 | Left | No fixation | 40/52 | 6/38 CCa | 32.50 | Down's syndrome |
| No op | Fix | 3/6 SiK |
BE, both eyes; CCa, Cardiff cards; CrK, crowded K; op, operation; pre‐op, preoperation; SiK, single K; Sn, Snellen; VA, visual acuity.
Median age at surgery, 17 weeks.
Results
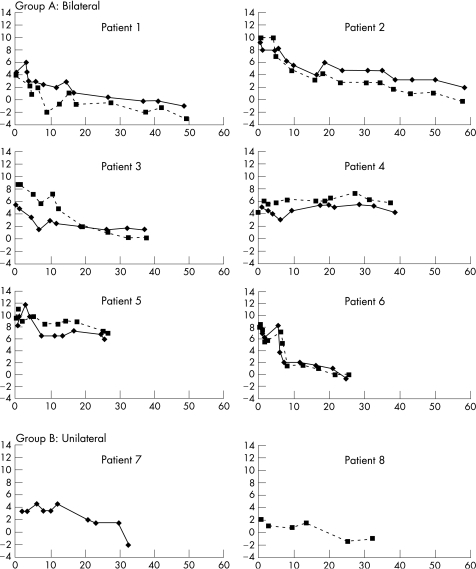
Eight children (14 eyes) were followed up for a median of 37.25 months (table 2). Figure 1 shows the PORC for each eye. No single transformation provided a good fit for all patients' data; some eyes exhibited a linear pattern of change over time, others logarithmic or exponential.
Figure 1 Mean sphere refraction (dioptres (D)) against time (x axis, months). Group A—bilateral, group B—unilateral. The solid line represents the right eye and the dotted line the left eye. Patients 1 and 2 had Pharmacia lens implants and patients 3–8 had Acrysof.
Patient 8 and patient 1(left eye) had their IOLs placed in the sulcus owing to surgical constraints. All lenses were well centred.
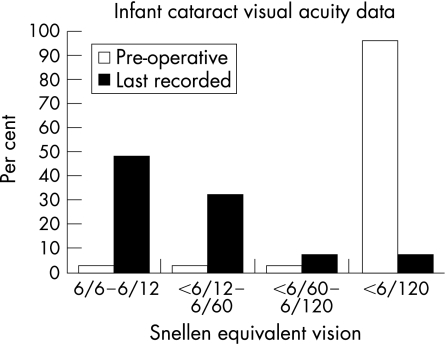
The visual acuity data (see table 2) is summarised in fig 2, the refractive results in table 2. The IOP was not increased >20 on any two successive occasions in any eye.
Figure 2 Recorded visual acuity preoperatively and at last follow‐up. See Hutchinson et al for comparison.8
Five patients required further surgery, usually removal of secondary lens matter (SLM) with or without further anterior vitrectomy. Patient 2 also needed enlarging of anterior and posterior capsulotomies. Patient 1 required esotropia surgery and removal of SLM. Five patients required seven EUAs.
Discussion
In total, 10 of the 14 eyes show myopic PORC over time; in 8 eyes the rate of PORC decreased with time (patients 1, 2, 3 and 6). The unilateral cases (Group B, case 8 with Down's syndrome) most closely follow a linear pattern of PORC. A previous publication demonstrated axial length growth was faster in infants with unilateral pseudophakia than in age‐matched controls with bilateral pseudophakia.9 This could contribute to the different pattern, however, in this case study these were also the eyes with the least postoperative hyperopia (POH).
Patients 1, 2, 3 and 6 could be demonstrating emmetropisation;alternatively, they could merely be showing an IOL choice accounting for a “pre‐determined” PORC. Cataract might conceivably interfere with emmetropisation; these eyes also have refractive overcorrection and patching of one eye (in seven of the eight children) for at least part of the study.
A strong inverse relationship was observed between POH and age at operation (partly explained by the “ceiling” of 30 D IOL and younger infants requiring higher IOL powers to achieve 6 D). The initial rate of PORC was not dependent on the POH despite published data suggesting faster axial length growth in infants operated at a younger age.10
Four eyes were myopic (−1.0 D sphere) at last follow‐up; the most myopic was −3.0 D sphere. Patient 1 had the second longest follow‐up; the other two eyes were the unilateral cases in Group B. These eyes had an initial POH of ⩽4.5 D sphere; a POH >4.5 D sphere may therefore be required to avoid early onset myopia. However, there is a degree of unpredictability between the refractive aim and POH as discussed below.
Four eyes of two patients (4 and 5) did not “emmetropise” or become myopic. Patient 4 initially showed hyperopic PORC. Patient 5 had microphthalmos, reflecting abnormal regulation of eye growth, which may explain a lack of emmetropisation. She also had nystagmus preoperatively. Both infants had poor vision preoperatively and good vision at final follow‐up. It is not clear why patient 4 had such a different pattern of PORC, but it shows a degree of uncertainty as to how refractions of any particular infant will change. In Group A, the PORC of both eyes has been similar in each infant, with the greatest final anisometropia in terms of spherical equivalent being 2.25 D.
Continued follow‐up may show a persistent drift towards myopia. The age at which emmetropia is reached may be more relevant as a target for IOL choice.
These rates of PORC are lower than those previously published for infants who were left aphakic11 (approximately 4.25 D sphere change by 36 months in this caseseries compared to over 9 D in aphakes).
A discrepancy was seen between SRK‐T refractive target and POH (median difference 0.65 D sphere; table 1), with a large range of error (approximately +/−3 D). Except for patient 2, the error was either hypermetropic or myopic for both eyes in Group A. If further substantiated, this could assist second eye IOL selection in future. Such errors are known to be more common in eyes with an axial length <20 mm, and in children <36 months. The error in the current study compares favourably with the error outlined in children <36 months.12 This error leads to uncertainty in IOL selection, but no formula satisfactorily resolves these accuracy issues.13
The visual outcomes of most eyes is encouraging within the limits of the follow‐up compared with data previously published in children undergoing surgery at a later age.5 Three patients had marked amblyopia at the end of the follow‐up of this series (patients 1, 3 and 8). Patients 1 and 3 (Group A) had nystagmus and very poor vision preoperatively, and dense amblyopia (2/30 and 1/30) at the end of follow‐up (the more amblyopic eye was not so preoperatively in either case—in one case it had been the first eye operated, in the other it had been the second). Patient 8 (Group B) has Down's syndrome and the amblyopic eye was the operated eye: the degree of amblyopia was less (6/38) than in the bilateral cases. Recent data suggest IOL implantation results in better visual acuity in unilateral cataract cases than does contact lens correction.14
Further follow‐up may show the possible long‐term risks of such early IOL implantation, in particular glaucoma. Tonopen accuracy in young children (with congenital glaucoma) has been questioned15 although the study suggests that overestimation is the main problem (>16 mm Hg) and therefore does not suggest that raised IOPs are likely to have been missed in the current study. These infants are at high risk of SLM obscuring their visual axis, particularly in the first 6–12 months after surgery.
In summary, when implanting an IOL in an infant <1 year of age for bilateral congenital cataracts, aiming for around +6 D sphere postoperatively or implanting a 30 D IOL where this target is unachievable using Acrysof lenses (ie, would require more than 30 D) usually leads to “emmetropisation” (at 2 –5 years of age in this case series). This assumes regular follow‐up, refraction and correction of refractive error and amblyopia. A proportion of cases may not show a myopic shift in this time.
No strong evidence is reported from this study that lens power selection for bilateral cataracts requires modification from that outlined, but for second eyes one should take account of any error for the first eye. The refractive target needs careful consideration in unilateral cataracts where PORC may at least initially follow a more linear pattern, and where anisometropia is more likely. PORC appears to be strongly linked to that of the other eye in infants who undergo bilateral cataract surgery, but variation between infants in how their refractive status varies with time has been demonstrated. Despite this variation, visual results can be excellent at the 3 years' follow‐up even with unexpected refractive outcomes (eg, patient 4).
The visual development of most of these eyes is encouraging, and with good postoperative management it seems that refractive variations may not have a considerable adverse effect on final vision. Further follow‐up of this and similar cohorts may help to optimise refractive and visual outcomes and make them increasingly predictable.
Abbreviations
EUA - examinations under anaesthetic
IOL - intraocular lens
POH - postoperative hyperopia
PORC - postoperative refractive change
SLM - secondary lens matter
Footnotes
Funding: None.
Competing interests: None declared.
References
- 1.Gordon R A, Donzis P B. Refractive development of the human eye. Arch Ophthalmol 1985103785–789. [DOI] [PubMed] [Google Scholar]
- 2.Fledelius H C, Christensen A C. Reappaisal of the human ocular growth curve in fetal life, infancy, and early childhood. Br J Ophthalmol 199680918–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Superstein R, Archer S M, Del Monte M A. Minimal myopic shift in pseudophakic versus aphakic paediatric cataract patients. JAAPOS 20026271–276. [DOI] [PubMed] [Google Scholar]
- 4.McClatchey S K, Parks M M. Theoretic refractive changes after lens implantation in childhood. Ophthalmologyl 19971041744–1751. [DOI] [PubMed] [Google Scholar]
- 5.Huthinson A K, Drews‐Botsch C, Lambert S R. Myopic shift after intraocular lens implantation during childhood. Ophthalmology 19971041752–1757. [DOI] [PubMed] [Google Scholar]
- 6.Peterseim M W, Wilson M E. Bilateral intraocular lens implantation in the paediatric population. Ophthalmology 20001071261–1266. [DOI] [PubMed] [Google Scholar]
- 7.Crouch E R, Crouch E R, Jr, Pressman S H. Prospective analysis of paediatric pseudophakia: myopic shift and postoperative outcomes. JAAPOS 20026277–282. [DOI] [PubMed] [Google Scholar]
- 8.Hutchinson A K, Drews‐Botsch C, Lambert S R. Myopic shift after intraocular lens implantation during childhood. Opthalmology 19971041752–1757. [DOI] [PubMed] [Google Scholar]
- 9.Vasavada A, Shetal M, Nihilani B. Rate of axial growth after congenital cataract surgery. Am J Ophthalmol 2004138915–924. [DOI] [PubMed] [Google Scholar]
- 10.Flitcroft D I, Knight‐Nanan D, Bowell R.et al Intraocular lenses in children: changes in axial length, corneal curvature, and refraction. Br J Ophthalmol 199983265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore B D. Changes in the aphakic refraction of children with unilateral congenital cataracts. J Pediatr Ophthalmolol Strabismus 198926290–295. [DOI] [PubMed] [Google Scholar]
- 12.Tromans C, Haigh P M, Biswas S.et al Accuracy of intraocular lens power calculation in paediatric cataract surgery. Br J Ophthalmol 200185939–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neely D E, Plager D A, Borger S M.et al Accuracy of intraocular lens calculations in infants undergoing cataract surgery. JAAPOS 20059160–165. [DOI] [PubMed] [Google Scholar]
- 14.Autrata R, Rehurek J, Vodckova K. Visual results after primary intraocular lens implantation or contact lens correction for aphakia in the first year of age. Ophthalmologica 200521972–79. [DOI] [PubMed] [Google Scholar]
- 15.Levy J, Lifshitz T, Rosen S.et al Is the tono‐pen accurate for measuring intraocular pressure in young children with congenital glaucoma? JAAPOS 20059321–325. [DOI] [PubMed] [Google Scholar]