Abstract
Background
Topical nitric oxide‐releasing dexamethasone (NCX1021) may avoid the negative effects of dexamethasone phosphate.
Aims
To obtain more information on the role of nitric oxide in glaucoma and to compare a nitric oxide‐releasing dexamethasone with dexamethasone phosphate with regard to intraocular pressure (IOP) and ocular haemodynamics in an experimental rabbit model.
Methods
Six rabbits were treated with dexamethasone phosphate 0.1% in the right eye and with NCX1021 in the left eye for 5 weeks. The parameters considered were IOP, nitric oxide marker levels in aqueous humour, ocular haemodynamics of ophthalmic artery (by means of colour Doppler imaging), expression of endothelial nitric oxide synthase (eNOS)in ciliary processes and histology of ciliary bodies.
Results
Dexamethasone increased IOP levels, NCX1021 did not. Nitrite and cyclic guanosine monophosphate levels in aqueous humour were lowered by dexamethasone and increased by NCX1021. Resistivity index of the ophthalmic artery was increased, eNOS expression was reduced and ciliary bodies showed histological lesions in dexamethasone‐treated eyes, not in NCX1021‐treated ones.
Conclusions
NCX1021 may avoid the IOP increase, impairment of ocular blood flow and the morphological changes in the ciliary bodies possibly induced by corticosteroid treatment.
Glaucoma is a progressive optic neuropathy characterised by loss of retinal ganglion cells, morphological changes in the optic nerve head and consequent visual field impairment. Many risk factors are considered to be associated with the pathogenesis of glaucoma. Ocular hypertension is considered to be the major risk factor for the development of this disease.1,2,3 Therefore, reduction in intraocular pressure (IOP) is actually the mainstay of treatment for glaucoma, but in some cases it is not sufficient to stop the progression of the disease.4,5,6
Among the vascular risk factors, several studies support the possible pathogenetic role of a dysfunction of the endothelium‐dependent vasoregulatory system.7,8,9 Two molecules released by endothelial cells have been characterised: endothelin 1, which has a vasoconstrictory effect, and nitric oxide, which has a vasodilative effect.10,11
Nitric oxide is a radical gas that is produced by the enzyme nitric oxide synthase (NOS) and has a very short half life. It is soluble in tissues and diffuses across membranes because of its small size. Nitric oxide is unstable; its measurable markers in biological fluids are nitrites (NO2−) and cyclic guanosine monophosphate (cGMP).11 It has a crucial role in complex processes such as vasodilatation, inflammation, thrombosis, immunity and neurotransmission.12,13
The different isoforms of NOS probably explain the multifaceted roles of nitric oxide in glaucoma pathogenesis. In fact, nitric oxide participates in outflow pathway, local modulation of ocular blood flow and apoptosis of retinal ganglion cells.14,15,16,17,18,19,20
Topical corticosteroids, the most useful drugs to treat ocular inflammatory diseases, may increase IOP by acting on the outflow pathway; therefore, their use is limited in patients with glaucoma.21
Nitric oxide donors have been shown to markedly reduce IOP in animals and humans.22,23 Nitric oxide‐donating steroids have been recently synthesised, with an aim to reduce the relevant side effects of steroids and possibly increase their activity.24 Wallerath et al25 showed in mice that systemic dexamethasone treatment increased arterial blood pressure in wild‐type endothelial nitric oxide synthase (eNOS)+/+ mice and not in eNOS−/− mice, showing that a down regulation of eNOS and a reduction in vascular NO production contribute to the vessel damage caused by corticosteroids.25
The presence of nitric oxide may reduce the effect of steroids in increasing IOP and may counterbalance their possible negative effects on the ocular vessels.
We conducted the present study to gain more information on the role of nitric oxide in glaucoma and to evaluate whether NCX1021, a topical nitric oxide‐releasing dexamethasone, would have different effects than dexamethasone on IOP, outflow pathway and ocular haemodynamics in a rabbit model of experimental corticosteroid‐induced glaucoma.
Materials and methods
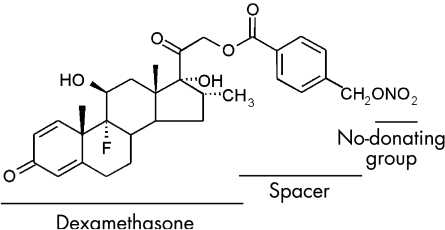
A nitric oxide‐releasing dexamethasone, NCX1021 (dexamethasone 21[4′‐(nitro‐oxymethyl)benzoate]), was compared with dexamethasone phosphate (fig 1). NCX1021 was recently synthesised at NicOx Laboratories, Milan, Italy.
Figure 1 Chemical structure of NCX1021: dexamethasone 21‐[21‐4′‐(nitro‐oxymethyl)benzoate].
The spacer between dexamethasone and the nitric oxide‐donating group assures the maintenance of the same chemical and pharmacological properties as dexamethasone phosphate.24
Six adult male New Zealand albino rabbits were studied. The experimental procedures conformed with those of the Declaration of Helsinki and with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the US National Institute of Health, and were conducted on authorisation of Italian regulations on protection of animals used for experimental and other scientific purposes (DM 116/1992) according to European Union regulations (OJ of ECL 358/1, 18 December 1986), and were approved by the local animal care Committee of the University of Florence, Florence, Italy.
The right eye of each rabbit was treated with dexamethasone phosphate 0.1% one drop three times a day for 5 weeks. The left eye was treated with NCX1021 following the same protocol.
The procedures listed below were carried out in constrained rabbits, using a topical anaesthesia with drops of benoxinate 0.4%.
IOP was measured in both eyes of all rabbits three times a day using Tono‐Pen XL (Solan Ophthalmic Products, Jacksonville, USA) by observers who were masked about the treatments; the mean value of three measurements was considered. Aqueous humour samples (200 μl) were withdrawn from the anterior chamber of each eye at baseline and then every week until the end of the study; the samples were treated with 3′‐isobutyl‐1‐methylxanthine to inhibit phosphodiesterase activity and immediately frozen at −80°C until use.
Haemodynamic evaluations were carried out by the same investigator, who was masked about the treatments, by means of a Color Doppler DynaView TM II SSD‐1700 (Aloka, Tokyo, Japan), using a 6‐MHz probe. In our personal experience, on the basis of unpublished data on the ocular blood flow evaluation in rabbits, this probe allows to obtain a good resolution and a high reproducibility, as regards the measurable parameters of retrobulbar haemodynamics. Systolic and diastolic velocities and resistivity index of the ophthalmic artery were evaluated in each eye at baseline, at week 2 of treatment and at the end of the study (fig 2).
Figure 2 Colour Doppler imaging of the ophthalmic artery.
At the end of the experiments the rabbits were killed with intravenous pentobarbital sodium.
The ciliary processes were isolated and prepared for the determination of eNOS protein and for morphological observations.
Nitrite concentrations in the aqueous humour were determined by the Griess reaction, evaluated by spectrophotometry and expressed as nanomoles per milligram of protein.
The concentration of cGMP was determined by means of a radioimmunoassay kit, using cGMP labelled with iodine‐125, and expressed as femtomoles per milligram of protein.26
Western blots were used to assess the expression of eNOS in ciliary processes. Ciliary bodies were prepared for western blot analysis, which was carried out with a mouse polyclonal antibody antagonist eNOS immunoglobulin (Ig)G1 1:1000.27 Densitometric analysis of the bands was carried out using the image analysis software (Image Pro‐Puis version 4.1, Media Cybernetics) of the National Institutes of Health, Bethesda, Maryland, USA. Protein content was determined with the Bradford method.26
Ciliary bodies were isolated from control rabbits, and those treated with dexamethasone or NCX1021. The ciliary bodies were fixed in an isotonic mixture of 0.6% formaldehyde and 0.5% acetic acid for 1 week at room temperature, dehydrated by graded ethanol and embedded in paraffin wax.28 For histopathological examinations, 5‐μm‐thick tissue sections were deparaffinised with xylene, stained with haematoxylin and eosin, and studied using light microscopy.
Results are shown as mean (standard error of mean (SEM)). Statistical comparisons between groups were carried out using Student's t test for paired observations. The one‐way analysis of variance was used to evaluate the parameters of retrobulbar haemodynamics; p<0.05 was considered to be significant. A semiquantitative evaluation was used to analyse the expression of eNOS in the ciliary processes; p<0.01 was considered to be significant.
Results
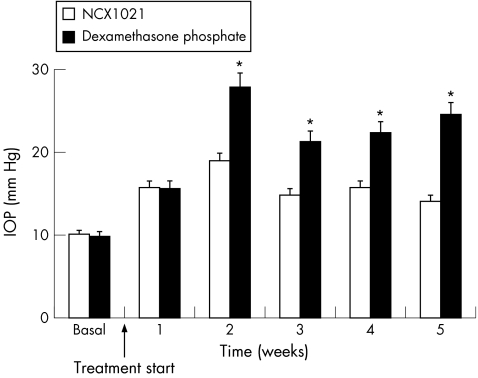
IOP increased after a few days of dexamethasone treatment, reaching the highest value at week 2 (basal, 9.8 (SEM 6); week 2, 27.8 (SEM 10) mm Hg). IOP values remained stably increased until the end of the treatment (24.5 (SEM 11) mm Hg at week 5). NCX1021 did not modify IOP levels especially during the last two weeks of the study (basal, 10.1 (SEM 4); week 2, 18.9 (SEM 4); and week 5, 14.1 (SEM 5) mm Hg). The difference between the two groups of eyes was significant from week 2 of treatment to the end of the follow‐up period (p<0.001; table 1, fig 3).
Table 1 Effect of dexamethasone phosphate and NCX1021 on intraocular pressure.
| Week | Intraocular pressure, mean (SEM) mm Hg |
|---|---|
| Week 0 (basal) | |
| NCX1021 | 10.1 (4) |
| Dexamethasone | 9.8 (6) |
| Week 1 | |
| NCX1021 | 15.2 (5) |
| Dexamethasone | 14.7 (7) |
| Week 2 | |
| NCX1021 | 18.9 (4) |
| Dexamethasone | 27.8 (10) |
| Week 3 | |
| NCX1021 | 13.3 (5) |
| Dexamethasone | 21.8 (8) |
| Week 4 | |
| NCX1021 | 15.1 (6) |
| Dexamethasone | 22.9 (10) |
| Week 5 | |
| NCX1021 | 14.1 (5) |
| Dexamethasone | 24.5 (11) |
Figure 3 Effect of dexamethasone phosphate and NCX1021 on intraocular pressure (IOP). White columns, NCX1021‐treated eyes; black columns, dexamethasone‐treated eyes. *p<0.001. See table 1 for data.
In dexamethasone‐treated eyes, nitrite levels decreased in aqueous humour (basal, 15.5 (SEM 3); week 2, 11 (SEM 1); and week 5, 10.5 (SEM 2) nmol/mg). On the other hand, in NCX1021‐treated eyes, these levels increased from week 2 (basal, 15.4 (SEM 4); week 2, 26.9 (SEM 3); week 5, 26.4 (SEM 4) nmol/mg). The difference between the two groups of eyes reached significance at every week of follow‐up (p<0.001; table 2; fig 4).
Table 2 Effect of dexamethasone phosphate and NCX1021 on nitrite levels in aqueous humour.
| Week | Nitrite level, mean (SEM) nmol/mg |
|---|---|
| Week 0 (basal) | |
| NCX1021 | 15.4 (4) |
| Dexamethasone | 15.5 (3) |
| Week 1 | |
| NCX1021 | 25.7 (3) |
| Dexamethasone | 10.4 (2) |
| Week 2 | |
| NCX1021 | 26.9 (3) |
| Dexamethasone | 11.1 (1) |
| Week 3 | |
| NCX1021 | 23.7 (4) |
| Dexamethasone | 10.9 (2) |
| Week 4 | |
| NCX1021 | 25.1 (3) |
| Dexamethasone | 10.8 (3) |
| Week 5 | |
| NCX1021 | 26.4 (4) |
| Dexamethasone | 10.5 (2) |
Figure 4 Effect of dexamethasone phosphate and NCX1021 on nitrite levels in aqueous humour. White columns, NCX1021‐treated eyes; black columns, dexamethasone‐treated eyes. *p<0.001. See table 2 for data.
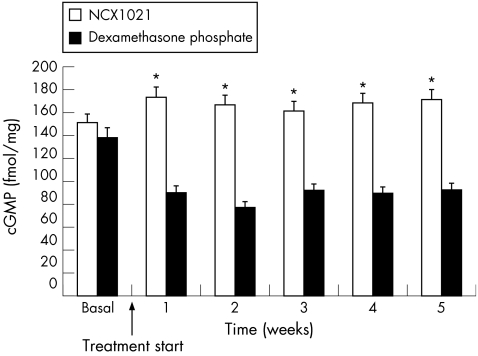
These findings were confirmed by the determination of cGMP levels in the aqueous humour, which were found to decrease in dexamethasone‐treated eyes (basal, 138.2 (SEM 43); week 2, 77.6 (SEM 17); and week 5, 92.7 (SEM 15) fmol/mg) and increase in NCX1021‐treated eyes (basal, 151.2 (SEM 24); week 2, 166.9 (SEM 33); and week 5, 171.6 (SEM 44) fmol/mg). The difference between the two groups of eyes was significant during the whole treatment period (p<0.001; table 3; fig 5).
Table 3 Effect of dexamethasone phosphate and NCX1021 on cyclic guanosine monophosphate levels in aqueous humour.
| Week | Cyclic guanosine monophosphate, mean (SEM) fmol/mg |
|---|---|
| Week 0 (basal) | |
| NCX1021 | 151.2 (24) |
| Dexamethasone | 138.2 (43) |
| Week 1 | |
| NCX1021 | 176.2 (38) |
| Dexamethasone | 87.4 (7.19) |
| Week 2 | |
| NCX1021 | 166.9 (33) |
| Dexamethasone | 77.6 (17) |
| Week 3 | |
| NCX1021 | 160.2 (31) |
| Dexamethasone | 88.9 (18) |
| Week 4 | |
| NCX1021 | 167.5 (34) |
| Dexamethasone | 86.3 (15) |
| Week 5 | |
| NCX1021 | 171.6 (64) |
| Dexamethasone | 92.7 (15) |
Figure 5 Effect of dexamethasone phosphate and NCX1021 on cyclic guanosine monophosphate (cGMP) levels in aqueous humour. White columns, NCX1021‐treated eyes; black columns, dexamethasone‐treated eyes. *p<0.001. See table 3 for data.
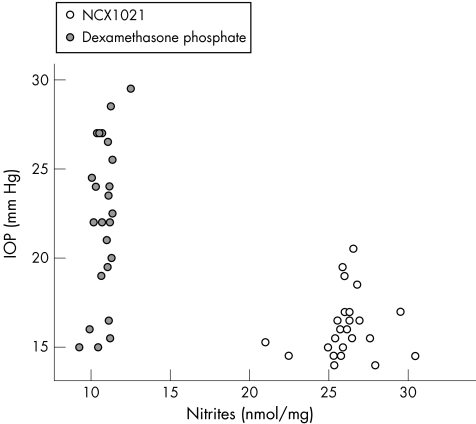
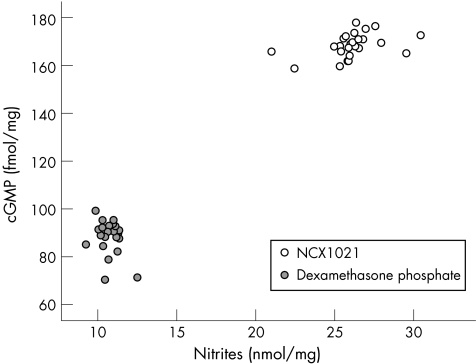
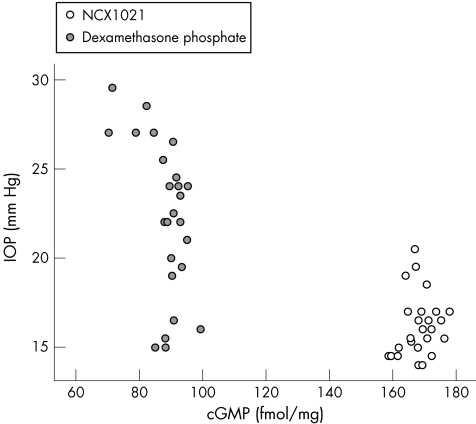
Figure 6 shows the relationship between IOP and nitrite levels, fig 7 that between nitrite and cGMP levels, and fig 8 shows the association between cGMP levels and IOP in dexamethasone‐treated and in NCX1021‐treated eyes.
Figure 6 Scatter plot of the two groups of eyes related to intraocular pressure (IOP) and nitrite levels in aqueous humour. Empty circles, NCX1021‐treated eyes; filled circles, dexamethasone‐treated eyes.
Figure 7 Scatter plot of the two groups of eyes related to cyclic guanosine monophosphate (cGMP) levels and nitrite levels in aqueous humour. Empty circles, NCX1021‐treated eyes; filled circles, dexamethasone‐treated eyes.
Figure 8 Scatter plot of the two groups of eyes related to intraocular pressure (IOP) and cyclic guanosine monophosphate (cGMP) levels in aqueous humour. Empty circles, NCX1021‐treated eyes; filled circles, dexamethasone‐treated eyes.
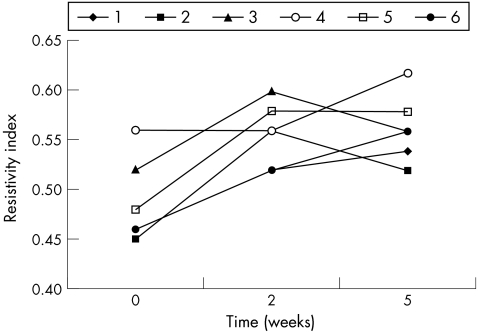
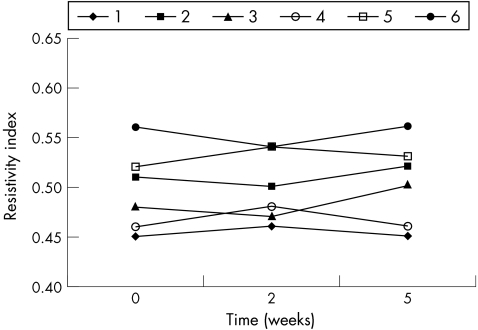
Resistivity index of ophthalmic artery, which was similar in the two groups at baseline, was increased in dexamethasone‐treated eyes at weeks 2 and 5 of treatment (p<0.001; table 4; fig 9). This parameter seemed not to be modified at any time in NCX1021‐treated eyes (p = 0.595; table 5; fig 10). Peak systolic velocity was not modified either in the dexamethasone‐treated (p = 0.390) or in the NCX1021‐treated (p = 0.546) eyes. We found no significant changes in end diastolic velocity between dexamethasone‐treated (p = 0.454) and NCX1021‐treated (p = 0.728) eyes.
Table 4 Resistivity index of the ophthalmic artery in dexamethasone phosphate‐treated eyes.
| Rabbit number | Resistivity index | ||
|---|---|---|---|
| Week 0 (basal) | Week 2 | Week 5 | |
| 1 | 0.46 | 0.52 | 0.54 |
| 2 | 0.45 | 0.56 | 0.52 |
| 3 | 0.52 | 0.60 | 0.56 |
| 4 | 0.56 | 0.56 | 0.62 |
| 5 | 0.48 | 0.58 | 0.58 |
| 6 | 0.46 | 0.52 | 0.56 |
Figure 9 Resistivity index of the ophthalmic artery in dexamethasone phosphate‐treated eyes (p<0.001). Each line corresponds to one rabbit. See table 4 for data.
Table 5 Resistivity index of the ophthalmic artery in NCX1021‐treated eyes.
| Rabbit number | Resistivity index | ||
|---|---|---|---|
| Week 0 (basal) | Week 2 | Week 5 | |
| 1 | 0.45 | 0.46 | 0.45 |
| 2 | 0.51 | 0.50 | 0.52 |
| 3 | 0.48 | 0.47 | 0.50 |
| 4 | 0.46 | 0.48 | 0.46 |
| 5 | 0.52 | 0.54 | 0.53 |
| 6 | 0.56 | 0.54 | 0.56 |
Figure 10 Resistivity index of the ophthalmic artery in NCX1021‐treated eyes (p<0.595). Each line corresponds to one rabbit. See table 5 for data.
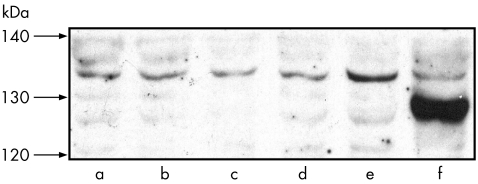
Western blot analysis showed that, in comparison to the values expressed in naive rabbits, dexamethasone decreased eNOS expression in ciliary processes, whereas NCX1021 did not (p<0.01; fig 11).
Figure 11 Western blot analysis of endothelial nitric oxide synthase (eNOS) protein expression in the ciliary body. Bands a and b, basal conditions; bands c and d, ciliary bodies of the eyes treated with dexamethasone phosphate; band e, ciliary bodies of the eyes treated with NCX1021; band f, marker.
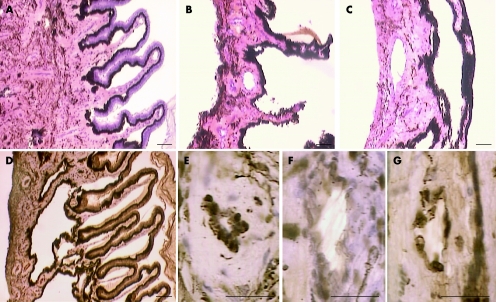
Examination of ciliary bodies by light microscopy showed a loss of epithelial cells, pigment reduction and thickening of the ciliary muscle in dexamethasone‐treated eyes. These lesions were not present in any NCX1021‐treated eyes (fig 12).
Figure 12 Light microscopy of ciliary bodies. (A,D,E) Histology of the ciliary bodies isolated from the NCX1021‐treated eyes. (B,C,F,G) Dexamethasone phosphate‐treated eyes.
Discussion
The present study supports the hypothesis, widely described in the literature, that nitric oxide has a multifaceted role in glaucoma pathogenesis. The present study focused on nitric oxide released by the endothelium, which is involved in the modulation of the outflow pathway and in retrobulbar haemodynamics. Nitric oxide synthesised by eNOS, differently from inducible NOS‐derived nitric oxide, seems not to be responsible for the formation of free radicals as peroxynitrites, which may be toxic for some ocular tissues.12,13,17
Our work suggests that NCX1021 may avoid the IOP increase, the impairment of retrobulbar circulation and the morphological changes in the ciliary bodies possibly induced by topical treatment with corticosteroids. Moreover, NCX1021 seems to be more effective than dexamethasone in reducing the inflammatory response due to neutrophils, by acting on the specific receptor for glucocorticoids on these cells (our unpublished observations).
According to our data, the release of nitric oxide seems to be implicated in avoiding ocular hypertension. Increased nitric oxide levels in the aqueous humour may avoid outflow impairment and prevent inhibition of nitric oxide synthesis by eNOS, two possible side effects of topical treatment with dexamethasone.
In our study, the resistivity index of ophthalmic artery was increased in dexamethasone‐treated eyes, whereas in NCX1021‐treated eyes, no noticeable modifications of this haemodynamic parameter were present. These data suggest that nitric oxide may have a positive effect in ocular haemodynamics too.
With respect to anti‐inflammatory response, NCX1021 maintains the same receptor of dexamethasone on activated neutrophils.
The lack of histopathological lesions of ciliary bodies could make NCX1021 a more profitable drug than dexamethasone.
In our study, we detected no changes in the lens during the whole follow‐up period. However, studies on rats and on humans suggest that inducible NOS might be responsible for cataractogenesis due to free radicals; there are no clear data about a cataractogenic role of eNOS.29,30 A few experimental studies have evaluated a possible cataractogenic role of topical steroids, but until now no certain data are available.31,32 The potential changes in the lens as a result of nitric oxide donors attached to topical steroid treatment have not been studied yet.
Further investigations are needed to confirm our preliminary data, which seem to be interesting from a clinical, therapeutical point of view. In fact, the opportunity to avoid dangerous effects of the topical treatment with corticosteroids may be relevant for patients with glaucoma and responders to corticosteroids.
Acknowledgements
We owe special thanks to Dr Daniele Bani, Department of Anatomy, Histology and Forensic Medicine, Section of Histology, University of Florence, Florence, Italy, for helping with the light microscopy of ciliary bodies.
Abbreviations
cGMP - cyclic guanosine monophosphate
eNOS - endothelial nitric oxide synthase
IOP - intraocular pressure
NOS - nitric acid synthase
SEM - standard error of mean
Footnotes
Competing interests: None.
References
- 1.Gordon M O, Beiser J A, Brandt J D.et al The Ocular Hypertension Treatment Study. Baseline factors that predict the onset of primary open‐angle glaucoma. Arch Ophthalmol 2002120714–720. [DOI] [PubMed] [Google Scholar]
- 2.Sommer A, Tielsch J M, Katz J. Relationship between intraocular pressure and primary open‐angle glaucoma among White and Black Americans. Arch Ophthalmol 19911091090–1095. [DOI] [PubMed] [Google Scholar]
- 3.Davenger M, Ringvold A, Bilka S. The probability of having glaucoma at different IOP levels. Acta Ophthalmol 199169565–568. [DOI] [PubMed] [Google Scholar]
- 4.Heijl A, Leske M C, Bengtsson B.et al Reduction of intraocular pressure and glaucoma progression. Results of the Early Manifest Glaucoma Trial. Arch Ophthalmol 20021201268–1279. [DOI] [PubMed] [Google Scholar]
- 5.The AGIS Investigators The Advanced Glaucoma Intervention Study (AGIS). VII: The relationship between control of IOP and visual field deterioration, Am J Ophthalmol 2000130429–440. [DOI] [PubMed] [Google Scholar]
- 6.Feiner L, Piltz‐Seymour J R. Collaborative Initial Glaucoma Treatment Study: a summary of results to date. Curr Opin Ophthalmol 200314106–111. [DOI] [PubMed] [Google Scholar]
- 7.Flammer J, Haefliger I O, Orgul S.et al Vascular dysregulation: a principal risk factor for glaucomatous damage? J Glaucoma 19998212–219. [PubMed] [Google Scholar]
- 8.Flammer J, Orgul S, Costa V P.et al The impact of ocular blood flow in glaucoma. Prog Retin Eye Res 200221359–393. [DOI] [PubMed] [Google Scholar]
- 9.Bonomi L, Marchini G, Marraffa M.et al Vascular risk factors for primary open angle glaucoma: the Egna–Neumarkt Study. Ophthalmology 20001071287–1293. [DOI] [PubMed] [Google Scholar]
- 10.Flammer J. Endothelin 1 in the pathogenesis of glaucoma. In: Drance SM, ed. Vascular risk factors and neuroprotection in glaucoma. The Hague: Kugler, 199797–103.
- 11.Palmer R M, Ferrige A G, Moncada S. Nitric oxide release accounts for the biological activity of endothelium‐derived relaxing factor. Nature 1987288373–376. [DOI] [PubMed] [Google Scholar]
- 12.Lowenstein C J, Dinerman J L, Snyder S H. Nitric oxide: a physiological messenger. Ann Intern Med 1994120227–237. [DOI] [PubMed] [Google Scholar]
- 13.Vallance P, Chan N. Endothelial function and nitric oxide: clinical relevance. Heart 200185342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nathanson J A, McKee M. Identification of an extensive system of nitric oxide‐producing cells in the ciliary muscle and outflow pathway of the human eye. Invest Ophthalmol Vis Sci 1995361765–1773. [PubMed] [Google Scholar]
- 15.Galassi F, Renieri G, Sodi A.et al Nitric oxide proxies and ocular perfusion pressure in primary open angle glaucoma. Br J Ophthalmol 200488757–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galassi F, Sodi A, Ucci F.et al Ocular hemodynamics and nitric oxide in normal pressure glaucoma. Acta Ophthalmol Scand200023237–38. [DOI] [PubMed] [Google Scholar]
- 17.Koss M C. Functional role of nitric oxide in regulation of ocular blood flow. Eur J Pharmacol 1999374161–174. [DOI] [PubMed] [Google Scholar]
- 18.Haefliger I O, Flammer J, Luscher T F. Nitric oxide and endothelin 1 are important regulators of human ophthalmic artery. Invest Ophthalmol Vis Sci 1992332340–2343. [PubMed] [Google Scholar]
- 19.Neufeld A H, Hernandez M R, Gonzales M. Nitric oxide synthase in the human glaucomatous optic nerve head. Arch Ophthalmol 1997115497–503. [DOI] [PubMed] [Google Scholar]
- 20.Quigley H A, Nickells R W, Kerrigan L A.et al Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Invest Ophthalmol Vis Sci 199536774–786. [PubMed] [Google Scholar]
- 21.Clark A F, Wilson K, McCartney M D.et al Glucocorticoid‐induced formation of cross‐linked actin networks in cultured human trabecular meshwork cells. Invest Ophthalmol Vis Sci 199435281–294. [PubMed] [Google Scholar]
- 22.Kotikoski H, Alajuuma P, Moilanen E.et al Comparison of nitric oxide donors in lowering intraocular pressure in rabbits: role of cyclic GMP. J Ocul Pharmacol Ther 20021811–13. [DOI] [PubMed] [Google Scholar]
- 23.Chuman H, Chuman T, Nao‐i N.et al The effect of L‐arginine on intraocular pressure in the human eye. Curr Eye Res 200020511–516. [PubMed] [Google Scholar]
- 24.Baraldi P G, Romagnoli R, Del Carmen Nunez M.et al Synthesis of nitro esters of prednisolone, new compounds combining pharmacological properties of both glucocorticoids and nitric oxide. J Med Chem 200447711–719. [DOI] [PubMed] [Google Scholar]
- 25.Wallerath T, Godecke A, Molojavyi A.et al Dexamethasone lacks effects on blood pressure in mice with a disrupted endothelial NO synthase gene. Nitric Oxide 20041036–41. [DOI] [PubMed] [Google Scholar]
- 26.Bradford M M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein‐dye binding. Anal Biochem 197672248–254. [DOI] [PubMed] [Google Scholar]
- 27.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970227680–685. [DOI] [PubMed] [Google Scholar]
- 28.Bianchi S, Mugnai L. Mast cell fixation and staining in image analysis. Eur J Basic Appl Histochem 199135161–174. [PubMed] [Google Scholar]
- 29.Ito Y, Nabekura T, Takeda M.et al Nitric oxide participates in cataract development in selenite‐treated rats. Curr Eye Res 200122215–220. [DOI] [PubMed] [Google Scholar]
- 30.Örnek K, Karel F, Büyükbingöl Z. May nitric oxide molecule have a role in the pathogenesis of human cataract? Exp Eye Res 20027623–27. [DOI] [PubMed] [Google Scholar]
- 31.Costagliola C, Iuliano G, Menzione M.et al Effect of glucocorticoid administration on the protein and nonprotein sulfhydryl groups of the rabbit lens. Ophthalmic Res 198719351–356. [DOI] [PubMed] [Google Scholar]
- 32.Shui Y B, Vrensen G F, Kojima M. Experimentally induced steroid cataract in the rat: a scanning electron microscope study. Surv Ophthalmol 199742S127–S132. [DOI] [PubMed] [Google Scholar]