Abstract
Purpose
To investigate the correlation of a structural measure of the macular area (optical coherence tomography (OCT)) with two functional measures (10‐2 Humphrey visual field (HVF) and multifocal visual evoked potential (mfVEP)) of macular function.
Methods
55 eyes with open‐angle glaucoma were enrolled. The 10‐2 HVF was defined as abnormal if clusters of ⩾3 points with p<5%, one of which had p<1%, were present. The mfVEP was abnormal if probability plots had ⩾2 adjacent points with p<1%, or ⩾3 adjacent points with p<5% and at least one of these points with p<1%. Two criteria were used for the macular OCT: (I) ⩾2 sectors with p<5% or 1 sector with p<1% and (II) 1 sector with p<5%.
Results
54 of the 55 eyes showed an abnormal 10‐2 HVF and 50 had central mfVEP defects. The two OCT criteria resulted in sensitivities of 85% and 91%. When both functional tests showed a defect (in 49 eyes), the OCT was abnormal in 45. For the OCT the outer and inner inferior regions were the most likely to be abnormal, and both functional techniques were most abnormal in the superior hemifield.
Conclusions
Good agreement exists between macular thickness and functional defects in patients with glaucoma. Study of the macular region may provide a quantitative measure for disease staging and monitoring.
Despite recent technological advances, the diagnosis of glaucoma is still based on visual field loss and the appearance of the optic disc. Some have suggested that in seeking early diagnosis of glaucomatous damage, it might be advantageous to assess the tissue loss in the perifoveal or macular region.1,2 Support for this comes from primate models of glaucoma, where considerable loss of retinal ganglion cells (RGC) occurs in the perifoveal region.3,4
The macular region is rich in RGC bodies and undergoes thinning in glaucoma.2,5 Whereas RGCs cannot yet be counted directly in vivo in humans, retinal thickness can be measured with many different techniques. Loss of retinal thickness can be used as a surrogate measure for the loss of RGC bodies and nerve fibre loss, as these layers contribute up to 40% of the entire retinal thickness in normal eyes.2,5
According to Leung et al,6 macular retinal thickness, as measured by optical coherence tomography (OCT), can detect glaucomatous damage and corresponds with peripapillary nerve fibre layer (NFL) thickness, a measure of RGC axons as well as glial cells. Thus, the question arises as to how this structural measure (macular thickness) of glaucomatous damage compares with functional measures of macular function. Here we compare OCT measures with two functional measures: the visual fields obtained with standard automated perimetry and the topographical information provided by the multifocal visual evoked potential (mfVEP). With the mfVEP technique, many (typically 60) responses, each associated with a local region of the visual field (or retina), are recorded simultaneously.7,8 Compared with other electrophysiological tests of visual function, the mfVEP has the advantage of producing a topographical measure of glaucomatous damage.8,9,10,11,12 Thus, mfVEP results can be compared with visual fields obtained with standard automated perimetry,13 as well as with structural measures.14
We investigated the extent to which the structural measure of the macular area (OCT) correlates with two functional measures (10‐2 HVF and mfVEP) of macular function.
Methods
Subjects
The study protocol was approved by the Institutional Review Board for Human Research of the New York Eye and Ear Infirmary, and informed consent was obtained from all subjects before their participation. Procedures adhered to the tenets of the Declaration of Helsinki.
A total of 42 patients (55 eyes) with glaucoma, ranging in age from 25 to 80 years (mean (SD) 62.6 (15.5) years), were prospectively enrolled from the Glaucoma Associates of New York (New York, New York, USA). The types of glaucoma were primary open‐angle (n = 20), normal‐tension (n = 14), pseudoexfoliation (n = 2), pigmentary (n = 3) and chronic closed‐angle (n = 3).
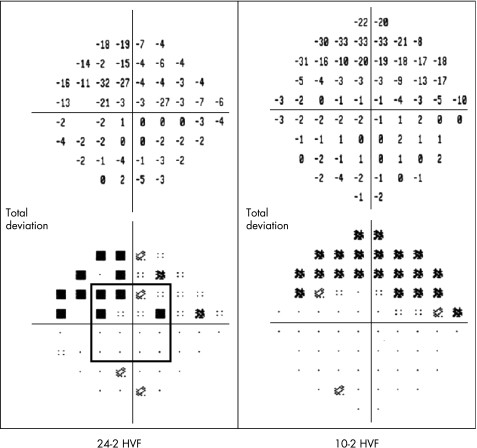
The inclusion criteria for an eye included visual acuity ⩾20/40, no clinical signs of macular disease, refractive error between ±6.00 diopters (D) spherical and ±3.00 D cylindrical, glaucomatous optic neuropathy and an abnormal 24‐2 HVF. An HVF was considered abnormal if an eye had an abnormal cluster in either hemifield that included the central field defined as ⩾3 contiguous points in the pattern deviation plot with a p value of <5%, with one at <1%. In addition, at least one of 16 central points had to be included in the cluster (four points adjacent to the fixation in each quadrant—fig 1). The mean (SD) deviation of the 24‐2 HVF was 9.4 (8) dB. Patients returned for 10‐2 HVF, mfVEP and OCT tests.
Figure 1 Humphrey visual field 24‐2 (left) and 10‐2 (right) patterns. Note the central 16 points (square) used for inclusion criteria.
Visual field testing
Achromatic automated perimetry was performed with a Humphrey Field Analyzer II (model 750, Carl Zeiss Meditec, Dublin, California, USA) using the 24‐2 and 10‐2 programme with either the full‐threshold or the SITA‐standard protocol. All patients had 24‐2 HVF tests as part of their standard ophthalmological examination. They returned for a 10‐2 HVF test.
Multifocal visual evoked potentials (mfVEP)
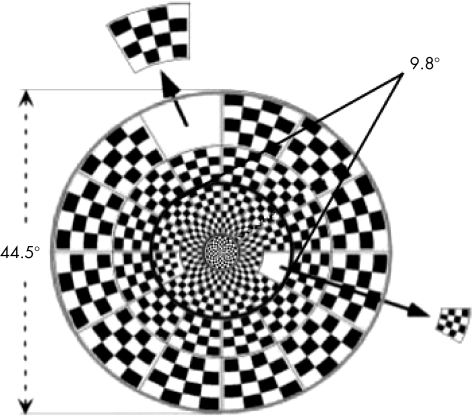
The mfVEP testing was performed on both eyes by a pattern reversal dartboard stimulus (fig 2) and the VERIS software from EDI (Electro‐Diagnostic Imaging, San Mateo, California, USA). The dartboard pattern consisted of 60 sectors, each with a checkerboard pattern of 16 checks: 8 white (200 cd/m2) and 8 black (<1 cd/m2). The sectors were scaled with eccentricity to stimulate roughly equal areas of the visual cortex.7 The entire display subtended a diameter of 44.5°, and the central 36 sectors fell within 9.8° of the foveal centre (fig 2).
Figure 2 The pattern reversal dartboard stimulus organised in three rings; 36 sectors are within the central 9.8°.
The details of the mfVEP recording and analysis have been published previously.8 Briefly, three channels of recording were obtained simultaneously with gold cup electrodes. The ground and reference electrodes were placed on the forehead and inion. One of the three active electrodes was placed 4 cm above the inion, and the other two were placed 1cm above and 4cm on either side of the inion. All three channels were filtered with a high‐frequency and a low‐frequency cut‐off of 3 Hz and 100 Hz, respectively (Grass Instruments preamplifier P511J, Quincy, Massachusetts, USA). An impedance of <5 K was achieved for all subjects. The mfVEPs were low‐pass filtered using a sharp cut‐off at 35 Hz and a fast Fourier transform technique. This and all other analyses were performed with programs written in MATLAB (Math‐Works, Natick, Massachusetts, USA). The exported mfVEP records were processed and an array of best channel responses derived as described previously.8,15,16,17 The amplitudes of each of the 60 local responses were compared with a normative group,18 and interocular (comparison of two eyes) and monocular probability plots, analogous to the HVF probability plots, were derived.8,15
Macular thickness measurements
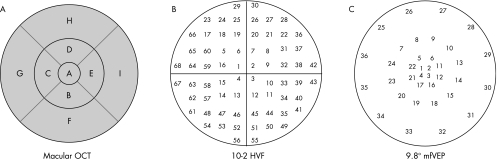
Macular thickness measurements were made with the Stratus OCT‐3 software V 4.0 (Carl Zeiss Meditec, Dublin, California, USA) and the fast macular thickness protocol. The macular scans consisted of six radial scans in a spoke‐like pattern centred on the fovea, with each radial scan spaced 30° from one another. The Stratus OCT software calculates retinal thickness as the distance between the vitreoretinal interface and the junction between the inner and outer segments of the photoreceptors. Three concentric circles divide the macular thickness map into three zones: fovea, inner macula and outer macula (fig 3A). The inner (B–E) and outer (F–I) zones are further divided into four quadrants by two diagonal lines. Thus, a total of nine regions (fovea, superior outer, superior inner, inferior outer, inferior inner, temporal outer, temporal inner, nasal outer and nasal inner) are available for analysis.19
Figure 3 (A) Macular optical coherence tomography (OCT) plot with the nine areas, (B) 10‐2 Humphrey visual field (HVF) plot numbered from 1 to 68 and (C) central 9.8° of the multifocal visual evoked potential (mfVEP) plot numbered from 1 to 36.
The quality of the scans was evaluated. Scans had to have focused images of the ocular fundus, a signal‐to‐noise ratio >33 dB, and had to be centred on the fovea. If one scan was classified as unacceptable, the patient was excluded from the study. The minimum pupil diameter was 3 mm.
Criteria for abnormal HVF, mfVEP and OCT tests
Cluster criteria
For the 10‐2 HVF, an eye was considered abnormal if there was an abnormal cluster in either hemifield. An abnormal cluster was defined as ⩾3 contiguous points in the pattern deviation plot with a p value of <5%, with at least one with a p value of <1%.
The mfVEP was considered abnormal if the interocular or monocular probability plots had an abnormal cluster defined as ⩾2 contiguous points with p values <1%, or ⩾3 contiguous points with p<5% and with at least one of these points with a p value <1%.
Two criteria were used for the macular OCT: (I) ⩾2 sectors with p<5% or 1 sector with p<1% and (II) 1 sector with p<5%.
Point or region criteria
In addition, each HVF or mfVEP point and each OCT region was classified as normal or abnormal according to whether the p value was ⩽5%.
Regional comparisons of the techniques
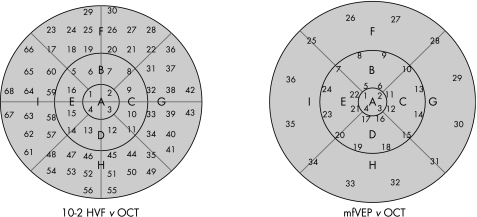
To make regional comparisons of these techniques, we had to take into consideration the fact that the spatial patterns of the three tests differed. Figure 3 shows the test regions of the macular OCT, 10‐2 HVF and mfVEP. In all three panels the outer circle has a radius of 10°. The nine regions of the OCT were labelled A–I (fig 3A) and the regions of the 10‐2 HVF and mfVEP 1–68 (fig 3B) and 1–36 (fig 3C), respectively. Note that the outer two rings of the mfVEP (fig 2) were omitted from fig 3A as they fell outside 10°. Figure 4 shows how we grouped the 10‐2 HVF (left panel) and mfVEP (right panel) test locations into regions compatible with those of the OCT.
Figure 4 10‐2 Humphrey visual field (HVF; left) and multifocal visual evoked potential (mfVEP; right) overlapping the optical coherence tomography (OCT) plot.
Results
On the basis of the cluster criteria, 54 of the 55 eyes (98%) showed an abnormal 10‐2 HVF, and 50 (91%) were abnormal on the mfVEP (central 10°). Two criteria were used for the macular OCT: (I) ⩾2 sectors with p<5% or 1 sector with p<1% and (II) 1 sector with p<5%. In all, 47 (85%) eyes had abnormal OCTs with Criterion I, and 50 (91%) eyes had abnormal OCTs with Criterion II. Overall, 45 (82%) of the 55 eyes analysed were abnormal on all three tests.
Table 1 shows the pairwise agreement among the three tests. Overall, the agreement between the functional and structural tests (85–89%) was nearly as good as that (89%) between the two functional tests.
Table 1 Number of abnormal and normal eyes.
| 10‐2 HVF | 10‐2 HVF | mfVEP | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | N | A | N | A | N | ||||||
| mfVEP | A | 49 | 1 | OCT (II) | A | 49 | 1 | OCT (II) | A | 46 | 4 |
| N | 5 | 0 | N | 5 | 0 | N | 4 | 1 | |||
| Agreement = 89% | Agreement = 89% | Agreement = 85% | |||||||||
A, abnormal; HVF, Humphrey visual field; mfVEP, multifocal visual evoked potential; N, normal; OCT, optical coherence tomography.
Table 2 indicates that when both functional tests (10‐2 HVF and mfVEP) were abnormal (49 eyes (89%)), the OCT was abnormal in 43 (88%) (Criterion I) and 45 (92%) (Criterion II) eyes—that is, in four (I) to six (II) eyes the OCT was normal even though both the functional tests were abnormal.
Table 2 Number of abnormal and normal eyes.
| 10‐2 HVF + mfVEP | |||
|---|---|---|---|
| A | N | ||
| OCT (I) | A | 43 | 0 |
| N | 6 | 0 | |
| Agreement = 88% | |||
| A | N | ||
| OCT (II) | A | 45 | 0 |
| N | 4 | 0 | |
| Agreement = 92% | |||
A, abnormal; HVF, Humphrey visual field; mfVEP, multifocal visual evoked potential; N, normal; OCT, optical coherence tomography.
For the macular OCT results, the regions F and B (68% and 49% of the eyes, respectively) were the most likely to be abnormal. The other regions ranged from 2% to 29%. To see if the abnormalities were more likely to appear in a particular part of the field on the 10‐2 HVF and mfVEP, we analysed these results after dividing the visual field into regions corresponding to the OCT (fig 4). In the case of the 10‐2 HVF, region F (fig 4, left panel), corresponding to the inferior outer macular thickness, was the most affected, with 78% of the points from all 55 eyes showing abnormality at the 5% level, and region B, corresponding to the inferior inner macular thickness, was the second most affected area (superior visual field). For the mfVEP, the same two areas were most affected, with the areas F and B showing 51% and 52% of the points as abnormal, respectively (table 3). Thus, on all three tests, the F and B regions showed the most abnormalities.
Table 3 Percentage of abnormal points within regions corresponding to the optical coherence tomography sectors.
| Abnormal points within each OCT sector (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | ||
| 10‐2 HVF | 38 | 65 | 39 | 35 | 49 | 78 | 51 | 43 | 58 | |
| mfVEP | 26 | 52 | 31 | 29 | 37 | 51 | 35 | 30 | 45 | |
HVF, Humphrey visual field; mfVEP, multifocal visual evoked potential; OCT, optical coherence tomography.
Discussion
We investigated the correlation between one structural (OCT) and two functional (10‐2 HVF and mfVEP) tests of the macula. To accomplish this, we selected eyes that we assumed had abnormal macular function, on the basis of the results of the 24‐2 HVF.
The 10‐2 HVF was abnormal in nearly all (98%) eyes. This was not surprising, as we included eyes that had at least one abnormal point out of the 16 central points on the 24‐2 HVF. The OCT and mfVEP were abnormal in 91% of the cases. Further, no eye was normal on both functional tests.
There was good agreement between the structural and functional measures. In fact, the macular OCT agreement with the 10‐2 HVF and mfVEP (85–89%) was as good as the agreement between the two functional tests. Of the four eyes in which the OCT did not agree with the functional tests, two (50%) had normal‐tension glaucoma; in total, 17 (30%) eyes had this type of glaucoma. Perhaps the macular thickness is influenced by intraocular pressure.
There was local agreement as well. The sectors showing more abnormalities on OCT showed more abnormal points on the functional tests. On OCT, the macular region F (outer inferior) was the most affected area, followed by region B (inner inferior). Consistent with these structural findings, both functional techniques showed greater defects in the superior hemifield. Thinning inferiorly has been reported to be the measure most strongly associated with glaucoma status in the NFL and optic nerve head.20,21,22
Structural damage to the optic nerve head and retinal nerve fibre layer (RNFL) can occur well before any detectable functional visual loss.23,24,25 In addition, the peripapillary RNFL sensitivity26,27,28 is reported to be higher than the macular thickness19,20,29 for discriminating between early to moderate glaucomatous and normal eyes. However, all studies comparing sensitivity and specificity between the optic nerve head and the macular region in patients with glaucoma used the 24‐2 HVF as the functional criterion. It seems better to compare the macular OCT with the 10‐2 HVF, as they are testing the same area. Further, macular thickness scanning is technically easier than NFL thickness scanning, the normal variability of cell density in the central retina being less than that in more peripheral areas5,20 and the reproducibility of macular thickness OCT measurements greater than that of NFL measurements.30 One important limitation of the macular OCT scan for glaucoma analysis is the choice of sectors. Sectors G, C, A, E and I include portions of the upper and lower hemifields (fig 3). Glaucomatous damage, on the other hand, often respects the midline (fig 1). Although in this study the agreement between the macular OCT and functional tests was very good, in general we would expect the best agreement if the OCT analysis respected the midline. However, currently this information is not readily available to the user.
Although previous studies showed that measurements of macular thickness are no better than measurements of peripapillary NFL thickness for early diagnosis of glaucoma,26,27,28 it is possible that macular testing might be better for monitoring progression. In any case, the OCT scans take relatively little time and there is no reason why both RNFL and macular scans cannot be performed as they provide different information. The health of the macula is more likely to correlate with the patient's quality of life, whereas the RNFL scans provide more global information. Further, the mechanisms and patterns of damage may well differ at these two sites.
In conclusion, our study shows good agreement between macular thickness and functional defects in patients with more advanced disease. The study of the macular region may provide a quantitative measure for disease staging and monitoring.
Acknowledgements
This study was supported by NIH/NEI Grant R01‐02115 and by the Shelley and Steven Einhorn Research Fund of the New York Glaucoma Research Institute, New York, NY. We thank the reviewer for his insightful comments.
Abbreviations
HVF - Humphrey visual field
mfVEP - multifocal visual evoked potential
NFL - nerve fibre layer
OCT - optical coherence tomography
RGC - retinal ganglion cells
RNFL - retinal nerve fibre layer
Footnotes
Competing interests: None.
References
- 1.Zeimer R, Shahidi M, Mori M.et al A new method for rapid mapping of the retinal thickness at the posterior pole. Invest Ophthalmol Vis Sci 1996371994–2001. [PubMed] [Google Scholar]
- 2.Zeimer R, Asrani S, Zou S.et al Quantitative detection of glaucomatous damage at the posterior pole by retinal thickness mapping. A pilot study. Ophthalmology 1998105224–231. [DOI] [PubMed] [Google Scholar]
- 3.Desatnik H, Quigley H A, Glovinsky Y. Study of central retinal ganglion cell loss in experimental glaucoma in monkey eyes. J Glaucoma 1996546–53. [PubMed] [Google Scholar]
- 4.Frishman L J, Shen F F, Du L.et al The scotopic electroretinogram of macaque after retinal ganglion cell loss from experimental glaucoma. Invest Ophthalmol Vis Sci 199637125–141. [PubMed] [Google Scholar]
- 5.Glovinsky Y, Quigley H A, Pease M E. Foveal ganglion cell loss is size dependent in experimental glaucoma. Invest Ophthalmol Vis Sci 199334395–400. [PubMed] [Google Scholar]
- 6.Leung C K, Chan W M, Yung W H.et al Comparison of macular and peripapillary measurements for the detection of glaucoma: an optical coherence tomography study. Ophthalmology 2005112391–400. [DOI] [PubMed] [Google Scholar]
- 7.Baseler H A, Sutter E E, Klein S A, Carney T. The topography of visual evoked response properties across the visual field. Electroencephalogr Clin Neurophysiol 19949065–81. [DOI] [PubMed] [Google Scholar]
- 8.Hood D C, Greenstein V C. Multifocal VEP and ganglion cell damage: applications and limitations for the study of glaucoma. Prog Retin Eye Res 200322201–251. [DOI] [PubMed] [Google Scholar]
- 9.Graham S L, Klistorner A I, Grigg J R.et al Objective VEP perimetry in glaucoma: asymmetry analysis to identify early deficits. J Glaucoma 2000910–19. [DOI] [PubMed] [Google Scholar]
- 10.Hood D C, Zhang X, Winn B J. Detecting glaucomatous damage with multifocal visual evoked potentials: how can a monocular test work? J Glaucoma 2003123–15. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg I, Graham S L, Klistorner A I. Multifocal objective perimetry in the detection of glaucomatous field loss. Am J Ophthalmol 200213329–39. [DOI] [PubMed] [Google Scholar]
- 12.Thienprasiddhi P, Greenstein V C, Chen C S.et al Multifocal visual evoked potential responses in glaucoma patients with unilateral hemifield defects. Am J Ophthalmol 200313634–40. [DOI] [PubMed] [Google Scholar]
- 13.Hood D C, Thienprasiddhi P, Greenstein V C.et al Detecting early to mild glaucomatous damage: a comparison of the multifocal VEP and automated perimetry. Invest Ophthalmol Vis Sci 200445492–498. [DOI] [PubMed] [Google Scholar]
- 14.Greenstein V C, Thienprasiddhi P, Ritch R.et al A method for comparing electrophysiological, psychophysical, and structural measures of glaucomatous damage. Arch Ophthalmol 20041221276–1284. [DOI] [PubMed] [Google Scholar]
- 15.Hood D C, Zhang X, Greenstein V C.et al An interocular comparison of the multifocal VEP: a possible technique for detecting local damage to the optic nerve. Invest Ophthalmol Vis Sci 2000411580–1587. [PubMed] [Google Scholar]
- 16.Hood D C, Zhang X, Hong J E.et al Quantifying the benefits of additional channels of multifocal VEP recording. Doc Ophthalmol 2002104303–320. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Hood D C, Chen C S.et al A signal‐to‐noise analysis of multifocal VEP responses: an objective definition for poor records. Doc Ophthalmol 2002104287–302. [DOI] [PubMed] [Google Scholar]
- 18.Fortune B, Zhang X, Hood D C.et al Normative ranges and specificity of the multifocal VEP. Doc Ophthalmol 200410987–100. [DOI] [PubMed] [Google Scholar]
- 19.Medeiros F A, Zangwill L M, Bowd C.et al Evaluation of retinal nerve fiber layer, optic nerve head, and macular thickness measurements for glaucoma detection using optical coherence tomography. Am J Ophthalmol 200513944–55. [DOI] [PubMed] [Google Scholar]
- 20.Guedes V, Schuman J S, Hertzmark E.et al Optical coherence tomography measurement of macular and nerve fiber layer thickness in normal and glaucomatous human eyes. Ophthalmology 2003110177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El Beltagi T A, Bowd C, Boden C.et al Retinal nerve fiber layer thickness measured with optical coherence tomography is related to visual function in glaucomatous eyes. Ophthalmology 20031102185–2191. [DOI] [PubMed] [Google Scholar]
- 22.Quigley H A, Addicks E M, Green W R.et al Optic nerve damage in human glaucoma. II. The site of injury and susceptibility to damage. Arch Ophthalmol 198199635–649. [DOI] [PubMed] [Google Scholar]
- 23.Bagga H, Greenfield D S. Quantitative assessment of structural damage in eyes with localized visual field abnormalities. Am J Ophthalmol 2004137797–805. [DOI] [PubMed] [Google Scholar]
- 24.Sommer A, Katz J, Quigley H A.et al Clinically detectable nerve fiber atrophy precedes the onset of glaucomatous field loss. Arch Ophthalmol 199110977–83. [DOI] [PubMed] [Google Scholar]
- 25.Quigley H A, Addicks E M, Green W R. Optic nerve damage in human glaucoma. III. Quantitative correlation of nerve fiber loss and visual field defect in glaucoma, ischemic neuropathy, papilledema, and toxic neuropathy. Arch Ophthalmol 1982100135–146. [DOI] [PubMed] [Google Scholar]
- 26.Sanchez‐Galeana C A, Bowd C, Zangwill L M.et al Short‐wavelength automated perimetry results are correlated with optical coherence tomography retinal nerve fiber layer thickness measurements in glaucomatous eyes. Ophthalmology 20041111866–1872. [DOI] [PubMed] [Google Scholar]
- 27.Budenz D L, Michael A, Chang R T.et al Sensitivity and specificity of the StratusOCT for perimetric glaucoma. Ophthalmology 20051123–9. [DOI] [PubMed] [Google Scholar]
- 28.Nouri‐Mahdavi K, Hoffman D, Tannenbaum D P.et al Identifying early glaucoma with optical coherence tomography. Am J Ophthalmol 2004137228–235. [DOI] [PubMed] [Google Scholar]
- 29.Wollstein G, Schuman J S, Price L L.et al Optical coherence tomography (OCT) macular and peripapillary retinal nerve fiber layer measurements and automated visual fields. Am J Ophthalmol 2004138218–225. [DOI] [PubMed] [Google Scholar]
- 30.Gurses‐Ozden R, Teng C, Vessani R.et al Macular and retinal nerve fiber layer thickness measurement reproducibility using optical coherence tomography (OCT‐3). J Glaucoma 200413238–244. [DOI] [PubMed] [Google Scholar]