Abstract
Objective
To determine whether earlier treatment of high‐risk, prethreshold retinopathy of prematurity (ROP) improves retinal structural outcome at 2 years of age.
Methods
Infants with bilateral high‐risk prethreshold ROP had one eye randomly assigned to treatment with peripheral retinal ablation. The fellow eye was managed conventionally, and either treated at threshold ROP or observed if threshold was never reached. In patients with asymmetrical disease, the high‐risk, prethreshold eye was randomised to earlier treatment or to conventional management. At 2 years of age, children were examined comprehensively by certified ophthalmologists to determine structural outcomes for their eyes. For the purposes of this study, an unfavourable structural outcome was defined as (1) a posterior retinal fold involving the macula, (2) a retinal detachment involving the macula or (3) retrolental tissue or “mass” obscuring the view of the posterior pole. Results of the 2‐year examination were compared with those from the 9 months examination.
Results
Data were available on 339 of 374 (90.6%) surviving children. Unfavourable structural outcomes were reduced from 15.4% in conventionally managed eyes to 9.1% in earlier‐treated eyes (p = 0.002) at 2 years of age. Ophthalmic side effects (excluding retinal structure) from the ROP or its treatment were similar in the earlier‐treated eyes and the conventionally managed eyes.
Conclusion
The benefit of earlier treatment of high‐risk prethreshold ROP on retinal structure endures to 2 years of age, and is not counterbalanced by any known side effect caused by earlier intervention. Earlier treatment improves the chance for long‐term favourable retinal structural outcome in eyes with high‐risk prethreshold ROP. Long‐term follow‐up is planned to determine structural and functional outcomes at 6 years of age.
The Early Treatment for Retinopathy Of Prematurity (ETROP) Study showed that retinal ablation for high‐risk prethreshold retinopathy of prematurity (ROP) improved structural and functional outcomes, compared with conventional management, when infants were examined at 9 months' corrected age.1 The study randomised infants who had both prethreshold disease and a risk for unfavourable structural outcome ⩾15%.2
However, eyes of infants may change over time.3 Myopia, strabismus and late retinal detachments all increase in frequency in the months and years after successful treatment of ROP.3,4 In the Cryotherapy for Retinopathy of Prematurity (CRYO‐ROP) Study, a wide distribution of optotype acuities developed after successful treatment, with 75% showing acuities worse than 20/40 when children reached 10 and 15 years of age.3,5 Time will tell whether the ETROP cohort will show the same distribution of functional outcomes as occurred in the CRYO‐ROP Study.
In the CRYO‐ROP Study, only a few infants had threshold disease in zone I,6 but in the ETROP Study, 40% of all randomised children had zone I disease. This group of children may be particularly vulnerable to complications of myopia, strabismus and late retinal detachment, but also to changes in other important aspects of visual functioning—for example, visual fields and contrast sensitivity. The reason for this is that a large area of avascular retina is ablated when zone I ROP is treated. Simply having disease in zone I, even when it regresses without treatment, could negatively affect visual fields.7 Recognising these potential important problems and the need to determine their frequency, the National Eye Institute extended funding to the ETROP Study to enable follow‐up on a yearly basis until children are 6 years old. All randomised children, including those with zone I or zone II disease, will be evaluated. Children are examined yearly by participating doctors to learn whether structural findings remain stable, but many functional outcomes cannot be measured until children are 6 years old. This report describes findings at the examination conducted when children were 2 years of age.
Methods
Study protocols were approved by the review boards of all participating institutions, and parents provided written informed consent before enrollment of their infants into the study and again at randomisation as additional consent for long‐term follow‐up was obtained. Details of the study design and laser technique are described previously.2
Infants with birth weights <1251 g and birth dates between 1 October 2000 and 30 September 2002 were screened at 26 participating centres. If at least one eye reached prethreshold ROP, the infant's demographic and ROP information was entered into the RM‐ROP2 risk model8 to determine the likelihood of progression to an unfavourable outcome in the absence of treatment. Details of the randomisation process have been described previously.2
The risk determination was made at the ETROP Coordinating Centre, School of Public Health, Coordinating Center for Clinical Trials, University of Texas Health Science Center, Houston, Texas, USA, using the RM‐ROP2 model to evaluate data provided by the clinical centre.8 If the risk of progression to an unfavourable outcome in the absence of treatment was calculated to be ⩾15%, consent for the randomised trial was obtained, and randomisation occurred. These eyes that had a risk of ⩾15% were termed as having high‐risk prethreshold and those with <15% risk were termed as having low‐risk prethreshold and were followed up every 2–4 days for at least 2 weeks until the ROP regressed or the risk progressed to ⩾15%. If both eyes were eligible for randomisation, one eye was assigned at random to earlier treatment with ablative therapy within 48 h of the first diagnosis of high‐risk prethreshold ROP. Treatment was generally laser therapy, but cryotherapy was permitted. The fellow eye served as the control and was managed conventionally, which meant that it was observed either until it reached threshold and was treated, or until the ROP regressed without progressing to threshold. In cases where only one eye had reached high‐risk prethreshold ROP, that eye was randomised to treatment within 48 h or to serve as a conventionally managed control, receiving treatment only if the ROP progressed to threshold severity. Infants in whom either eye had developed threshold ROP before randomisation were excluded from the study.
For the analyses at 6 and 9 months, and also for this 2‐year analysis, eyes in the randomised group were classified according to the zone and stage of ROP that were present at the time of randomisation. Results of examinations at 6 and 9 months' corrected age have been reported previously.1,9
When study participants reached 2 years of age, structural outcome was documented with a fundus examination through a dilated pupil by study‐certified examiners. The examination was carried out after instilling 1% cyclopentolate hydrochloride. When there was a medical contraindication to this drop, either 0.5% cyclopentolate or 1% tropicamide was used. An unfavourable outcome was defined as (1) a posterior retinal fold involving the macula, (2) a retinal detachment involving the macula or (3) retrolental tissue or mass obscuring the view of the posterior pole. Eyes that had received a vitrectomy or scleral buckle were classified for study purposes as having an unfavourable structural outcome.
Results
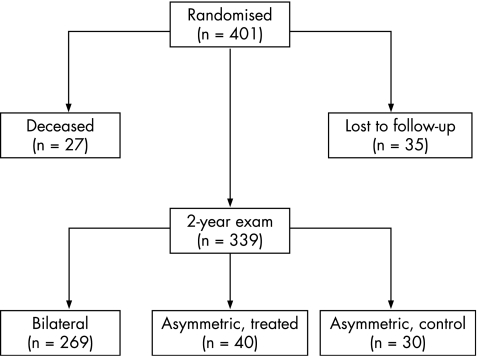
Structural outcome data were obtained from 339 of 374 (90.6%) surviving children at 2 years of age (fig 1). These are children who had at least one high‐risk prethreshold eye and who entered into the randomised portion of the study. In 269 of the children, high‐risk prethreshold ROP was present in both eyes (bilateral cases) at the time of randomisation, whereas in 70 children, the disease was asymmetrical (one eye was high‐risk prethreshold). Two‐year data were not obtained from 27 infants who died before the examination and 35 infants whose parents did not bring them in for the examination. Average corrected age at the 2‐year examination was 25.4 (standard deviation 2.7) months.
Figure 1 Structural outcome data from 339 of 374 surviving children at 2 years of age.
Table 1 gives the results for the 2‐year examination. Data indicate a significant benefit of treatment of eyes with high‐risk prethreshold ROP, with unfavourable structural findings reduced from 15.4% in conventionally managed eyes to 9.1% in high‐risk prethreshold treated eyes (p = 0.002). Results from infants with bilateral disease with discordant outcomes in the two eyes provide strong evidence of a beneficial effect of treatment of high‐risk prethreshold eyes.
Table 1 Two‐year structural outcome for randomised patients.
| Eyes treated at high‐risk prethreshold* | Conventionally managed eyes* | χ2 | p Value | |
|---|---|---|---|---|
| Bilateral | 269 (10.4) | 269 (16.7) | 8.76† | 0.003 |
| Asymmetric | 38 (0)‡ | 30 (3.3) | 1.29 | 0.26 |
| Total | 307 (9.1) | 299 (15.4) | 9.66 | 0.002 |
*Values are n (% unfavourable).
†Based on discordant pairs (25 infants with favourable outcomes in earlier‐treated eyes and unfavourable outcomes in conventionally managed eyes; 8 infants with unfavourable outcomes in earlier‐treated eyes and favourable outcomes in conventionally managed eyes.
‡Less than 40 because of inability to determine the structural outcome in 2 eyes (poor cooperation).
Table 2 shows the structural outcomes for randomised eyes, stratified by the International Classification of Retinopathy Of Prematurity category and by RM‐ROP2 risk category. The greatest benefit of treatment at high‐risk prethreshold ROP versus conventional management occurred in eyes that had zone I, stage 3 ROP, with and without plus (unfavourable structural outcomes in 25.0% early treated versus 58.3% conventionally managed eyes). A relative benefit from intervention at high‐risk prethreshold ROP for structural outcomes was also seen among eyes that had zone I, stage 1 or 2 ROP without plus disease, and among eyes that had zone II, stage 3 ROP with plus disease.
Table 2 Structural outcome at 2 years for infants with bilateral high‐risk prethreshold retinopathy of prematurity by the International Classification of Retinopathy Of Prematurity category and Risk Management of Retinopathy Of Prematurity 2 risk.
| ICROP classification | Eyes treated at high‐risk prethreshold | Conventionally managed eyes | Discordant pairs | |||
|---|---|---|---|---|---|---|
| Zone | Stage | Plus disease | n% UF | n% UF | A* | B† |
| I | 3 | Yes or no | 24 (25.0) | 24 (58.3) | 8 | 0 |
| I | 1 or 2 | Yes | 9 (22.2) | 9 (22.2) | 0 | 0 |
| I | 1 or 2 | No | 71 (4.2) | 71 (8.5) | 5 | 2 |
| II | 3 | Yes | 101 (8.9) | 101 (11.9) | 7 | 4 |
| II | 3 | No | 3 (0.0) | 3 (0.0) | 0 | 0 |
| II | 2 | Yes | 30 (20.0) | 30 (16.7) | 1 | 2 |
| RM‐ROP2 risk | ||||||
| 0.15–0.30 | 104 (7.7) | 104 (7.7) | 4 | 4 | ||
| 0.30–0.45 | 69 (8.7) | 69 (14.5) | 6 | 2 | ||
| ⩾0.45 | 72 (18.1) | 72 (33.3) | 13 | 2 | ||
UF, unfavourable.
ICROP, International Classification of Retinopathy Of Prematurity; RM‐ROP, Risk Management of Retinopathy Of Prematurity.
*For group A, earlier‐treated eyes had a favourable outcome, and conventionally managed eyes had an unfavourable outcome.
†For group B, earlier‐treated eyes had an unfavourable outcome, and conventionally managed eyes had a favourable outcome.
As shown at the bottom of table 2, examination of outcome by RM‐ROP2 risk category showed greater benefit for structural outcomes for earlier treatment in high‐risk prethreshold eyes with ⩾30% risk than in high‐risk prethreshold eyes with 15 to <30% risk.
Table 3 summarises the ocular findings among treated high‐risk prethreshold eyes versus conventionally managed high‐risk prethreshold eyes that progressed and later underwent treatment at threshold ROP or underwent involution of ROP without requiring treatment. Ocular complication rates were similar in the two groups. Note that at 2 years of age, cataract or aphakia was found in 11 (4.1%) treated eyes and in 16 (6.0%) conventionally managed eyes.
Table 3 Summary of ocular findings observed by the examining physician in 2‐year‐old randomised children who had bilateral high‐risk, prethreshold retinopathy of prematurity.
| Variables | Sample size | % Present* | % Unable to assess | Discordant pairs† | p Value‡ | ||
|---|---|---|---|---|---|---|---|
| Earlier‐treated eye | Conventionally managed eye | Earlier‐treated eye | Conventionally managed eye | ||||
| Corneal opacification | 269 | 1.5 | 2.6 | 0 | 0.7 | 6/3 | 0.51 |
| Amblyopia | 269 | 5.9 | 10.8 | 0 | 0 | 27/14 | 0.06 |
| Anterior segment | |||||||
| Synechiae | 269 | 1.9 | 1.9 | 1.5 | 2.2 | 4/4 | 1.00 |
| Cataract or aphakia | 269 | 4.1 | 6.0 | 0.7 | 1.1 | 12/7 | 0.36 |
| Glaucoma | 269 | 0.7 | 1.5 | 0.0 | 0.7 | 3/1 | 0.63 |
| Extensive vitreous membranes/org | 269 | 2.6 | 4.2 | 0.4 | 1.5 | 9/5 | 0.42 |
| Fundus | |||||||
| Optic nerve‐pallor (part/severe) | 269 | 7.4 | 9.3 | 4.8 | 8.2 | 4/2 | 0.69 |
| Optic nerve cup:disc ratio ⩾0.5 | 269 | 7.8 | 10.1 | 5.2 | 8.2 | 3/0 | 0.25 |
| Straightened vessels in zone I | 269 | 17.9 | 18.1 | 4.5 | 7.4 | 18/12 | 0.36 |
| Macular ectopia | 269 | 5.4 | 4.8 | 4.1 | 7.4 | 7/6 | 1.00 |
| Foveal pigmentation disturbance | 269 | 5.4 | 5.6 | 4.5 | 7.8 | 4/4 | 1.00 |
| Retinal fold | 269 | 2.3 | 3.2 | 3.0 | 5.9 | 7/3 | 0.34 |
| Retrolental membrane | 269 | 2.6 | 3.4 | 1.5 | 3.0 | 7/4 | 0.55 |
| Retinal breaks or tears (periphery) | 269 | 0.4 | 0.4 | 4.1 | 8.2 | 0/1 | 1.00 |
| Pre‐retinal membranes (periphery) | 269 | 4.2 | 5.6 | 3.3 | 7.1 | 7/4 | 0.55 |
| Detachment or retinoschisis | 269 | 3.8 | 6.2 | 2.2 | 4.1 | 11/5 | 0.21 |
*Percent of those that were able to determine.
†Condition absent or normal in treated eye but not in control eye, or condition present or abnormal in treated eye but not in control eye.
‡p Values calculated using binomial probability distribution.
Table 4 shows the distribution of possible anatomical outcomes in the earlier‐treated eyes and conventionally managed eyes. Table 5 compares structural outcome data from examinations at 6 months, 9 months and 2 years. Unfavourable structural outcomes increased from 6 to 9 months as a result of reclassification of 4A detachments that were treated with vitrectomy or buckling (see footnote to table 5). Results for structural outcomes from 9 months to 2 years are remarkably consistent.
Table 4 Two‐year structural outcomes.
| Two‐year outcomes | Earlier‐treated eyes | Conventionally managed eyes |
|---|---|---|
| Normal | 240 | 219 |
| Abnormal angle of temporal retinal vessels | 28 | 25 |
| Macular heterotopia | 11 | 8 |
| Retinal detachment stage* | ||
| 4A | 0 | 1 |
| 4B | 1 | 1 |
| 4C | 4 | 0 |
| 5A | 3 | 2 |
| 5B | 0 | 1 |
| 6A, 6B | 0 | 1 |
| Buckling procedure | 4 | 6 |
| Vitrectomy | 16 | 35 |
| Unable to grade | 2 | 0 |
| Total | 309 | 299 |
*4A retinal detachment, retinoschisis or fold in the near periphery (sparing fovea); 4B, retinal detachment, retinoschisis or fold involving fovea; 4C, view of macula (and presumably patient's central vision) blocked as a result of partial cataract or partial retrolental membrane or partial corneal opacity (due to retinopathy of prematurity (ROP)); 5A, total retinal detachment or retinoschisis, or total retrolental membrane; 5B, all view of posterior pole and near periphery is blocked as a result of total cataract or total corneal opacity (due to ROP); 6A, enucleation due to ROP; 6B, enucleation due to other causes.
Table 5 Structural outcomes over time.
| Age | Earlier‐treated eyes | Conventionally managed eyes | χ2 | p Value | Discordant pairs | ||
|---|---|---|---|---|---|---|---|
| n | Unfavourable (%)* | n | Unfavourable (%)* | ||||
| 6 months | 325 | 4.9 | 319 | 10.0 | 9.2 | 0.002 | 21/6 |
| 9 months† | 332 | 9.3 | 327 | 15.6 | 11.0 | <0.001 | 25/7 |
| 2 years | 307 | 9.1 | 299 | 15.4 | 9.66 | 0.002 | 25/8 |
*Percentage of eyes with unfavourable structural outcome increased between 6 and 9 months. 4A detachments before vitrectomy or buckling surgery were classified as favourable at 6 months. If an eye with a retinal detachment grading 4A then had vitrectomy or buckling surgery, it was reclassified by the protocol as unfavourable at 9 months.
†Numbers differ slightly from published final results8 because one additional child that underwent a vitrectomy before 9 months of age was not reported until after the date of the publication.
Discussion
Infants randomised into the ETROP Study differ from infants in the CRYO‐ROP Study in considerable ways, so it cannot be assumed that long‐term complications and outcomes will be the same for the ETROP cohort of children. In the ETROP Study, one eye of randomised infants was treated at an earlier point in the disease process at prethreshold. Earlier treatment reduced the rate of unfavourable structural and functional outcomes, but the question arises as to whether earlier treatment could increase or decrease the risk of later complications. Adding to this question is the fact that the ETROP cohort is comprised of a large percentage of children with zone I prethreshold ROP eyes. The extensive treatment required to manage ROP in zone I required ablation of large areas of retina. The amount of treatment in these infants could affect stability of structural and functional status. Treatment was administered with a laser in most cases, which also distinguishes this cohort from the CRYO‐ROP cohort, where retinal ablation with cryotherapy was used.10
Furthermore, eyes of infants from the ETROP Study were randomised according to ROP risk status, and therefore the ETROP cohort consists of selected children with particularly vulnerable eyes compared with the CRYO‐ROP Study cohort.2 For example, the average birth weight of infants in the ETROP Study was 100 g less than that of the CRYO‐ROP Study infants. Other risk factors such as speed of progression of ROP disease, ethnicity of the child and time of onset of ROP could affect various outcome measures for eyes in the ETROP Study.11
It is therefore reassuring that the retinal structural benefit of earlier treatment for high‐risk prethreshold ROP persists from 9 months to 2 years. This finding indicates that structural findings remain constant through the first 2 years of age. The 2‐year examination does not include any functional component (eg, visual acuity), but the high correlation between structure and function found in previous ROP trials suggests that as a group, children's eyes that underwent retinal ablation for high‐risk prethreshold ROP may have better functional outcomes than children's eyes with high‐risk prethreshold ROP that were managed conventionally.1,3,5,6 The functional outcome will be determined at 6 years of age.
Side effects of treatment are also similar for both the earlier‐treated and conventionally managed eyes. Complications of cataract, glaucoma and corneal damage are the same for both groups of eyes. Fundus changes, including optic nerve atrophy and foveal pigment disturbances, are also similar for the two groups of eyes.
Visual function at 2 years of age usually cannot be measured quantitatively with recognition acuity optotypes. Visual acuity measured with optotypes, contrast sensitivity and visual field extent will be measured at subsequent examinations, when children are old enough to cooperate with the test procedures. Arguably, infants who had high‐risk prethreshold ROP in zone I are at particular risk for later complications and diminished function. With 40% of the ETROP cohort managed surgically in at least one eye for zone I disease, the concern is that gains in structural status may be offset to some degree by a diminution of visual field or even visual acuity. For this reason, the ETROP study has carefully distinguished those eyes that should be offered earlier treatment from those in which careful observation is indicated, with treatment recommended if progression occurs.1,12 Functional outcomes in this cohort will be the subject of future reports, as children who were randomised in the ETROP Study grow older.
Abbreviations
CRYO‐ROP - Cryotherapy for Retinopathy Of Prematurity
ETROP - Early Treatment for Retinopathy Of Prematurity
ROP - retinopathy of prematurity
Footnotes
Funding: This study was supported by cooperative agreements (5U10 EY12471 and 5U10 EY12472) with the National Eye Institute of the National Institutes of Health, US Department of Health and Human Services, Bethesda, Maryland.
References
- 1.The Early Treatment for Retinopathy of Prematurity Cooperative Group Revised indications for treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol 20031211684–1696. [DOI] [PubMed] [Google Scholar]
- 2.The Early Treatment for Retinopathy of Prematurity Cooperative Group Multicenter trial of early treatment for retinopathy of prematurity: study design. Control Clin Trials 200425311–325. [DOI] [PubMed] [Google Scholar]
- 3.Cryotherapy for Retinopathy of Prematurity Cooperative Group 15‐year outcomes following threshold retinopathy of prematurity: final results from the multicenter trial of cryotherapy for retinopathy of prematurity. Arch Ophthalmol 2005123311–318. [DOI] [PubMed] [Google Scholar]
- 4.Quinn G E, Dobson V, Repka M X.et al Development of myopia in infants with birth weights of less than 1251g. Ophthalmology 199299329–340. [DOI] [PubMed] [Google Scholar]
- 5.Cryotherapy for Retinopathy of Prematurity Cooperative Group Multicenter trial of cryotherapy for retinopathy of prematurity: ophthalmological outcomes at 10 years. Arch Ophthalmol 20011191110–1118. [DOI] [PubMed] [Google Scholar]
- 6.Cryotherapy for Retinopathy of Prematurity Cooperative Group Multicenter trial of cryotherapy for retinopathy of prematurity: preliminary results. Arch Ophthalmol 1988106471–479. [DOI] [PubMed] [Google Scholar]
- 7.Quinn G E, Dobson V, Hardy R J.et al Visual fields measured with double‐arc perimetry in eyes with threshold retinopathy of prematurity (ROP) from the CRYO‐ROP trial. Ophthalmology 19961031432–1437. [DOI] [PubMed] [Google Scholar]
- 8.Hardy R J, Palmer E A, Dobson V.et al Risk analysis of prethreshold ROP. Arch Ophthalmol 20031211697–1701. [DOI] [PubMed] [Google Scholar]
- 9.Good WV on behalf of the Early Treatment for Retinopathy of Prematurity Cooperative Group Final results of the early treatment for retinopathy of prematurity (ETROP) randomized trial. Trans Am Ophthalmol Soc 2004102233–250. [PMC free article] [PubMed] [Google Scholar]
- 10.Cryotherapy for Retinopathy of Prematurity Cooperative Group Multicenter trial of cryotherapy for retinopathy of prematurity: one year outcome—structure and function. Arch Ophthalmol 19901081408–1416. [PubMed] [Google Scholar]
- 11.Early Treatment for Retinopathy of Prematurity Cooperative Group The incidence and course of retinopathy of prematurity: findings from the early treatment for retinopathy of prematurity study. Pediatrics 200511615–23. [DOI] [PubMed] [Google Scholar]
- 12.Good W V, Hardy R J. The dilemma of exercising clinical judgment in the treatment of retinopathy of prematurity—reply. Arch Ophthalmol 2005123409–410. [DOI] [PubMed] [Google Scholar]