Abstract
In cultured cerebrocortical neurons, mild excitotoxic insults or staurosporine result in apoptosis. We show here that N-methyl-d-aspartate (NMDA) receptor-mediated, but not staurosporine-mediated, apoptosis is preceded by depolarization of the mitochondrial membrane potential (Δψm) and ATP loss. Both insults, however, release cytochrome c (Cyt c) into the cytoplasm. What prompts mitochondria to release Cyt c and the mechanism of release are as yet unknown. We examined the effect of inhibition of the adenine nucleotide translocator (ANT), a putative component of the mitochondrial permeability transition pore. Inhibition of the mitochondrial ANT with bongkrekic acid (BA) prevented NMDA receptor-mediated apoptosis of cerebrocortical neurons. Concomitantly, BA prevented Δψm depolarization, promoted recovery of cellular ATP content, and blocked caspase-3 activation. However, in the presence of BA, Cyt c was still released. Because BA prevented NMDA-induced caspase-3 activation and apoptosis, the presence of Cyt c in the neuronal cytoplasm is not sufficient for the induction of caspase activity or apoptosis. In contrast to these findings, BA was ineffective in preventing staurosporine-induced activation of caspases or apoptosis. Additionally, staurosporine-induced, but not NMDA-induced, apoptosis was associated with activation of caspase-8. These results indicate that, in cerebrocortical cultures, excessive NMDA receptor activation precipitates neuronal apoptosis by means of mitochondrial dysfunction, whereas staurosporine utilizes a distinct pathway.
Apoptosis is an important mechanism in both the development and degeneration of the nervous system. Evidence suggests that the loss of neurons in many neurologic disorders occurs by apoptosis (1–4). The overstimulation of glutamate receptors can precipitate the death of neurons by either necrosis or apoptosis depending on the severity of the insult (5, 6). Activation of the N-methyl-d-aspartate (NMDA) subtype of glutamate receptor, in particular, results in an increase in the intracellular free calcium concentration ([Ca2+]i) to levels above the buffering capacity of neurons (7). This increase in [Ca2+]i leads to the activation of toxic events, including oxidative and nitrosative stress (8). Mitochondria, which undergo harmful Ca2+-loading after NMDA receptor activation (9, 10), also have an important signaling function in apoptosis (11–13).
Apoptotic cell death is often mediated by a caspase cascade. Although many stimuli exist, the final phases of apoptosis are executed by a few common effector caspases. Mitochondria appear to provide a link between the initiator caspases and the downstream effector caspases. In nonneuronal cells, mitochondria have been shown to accelerate activation of caspases by releasing proapoptotic molecules, such as cytochrome c (Cyt c) (11, 12) and the apoptosis-inducing factor (13). The release of these molecules can be stimulated by some caspases and by Bid and Bax (14–16), whereas Bcl2 prevents their release (11–13).
The mechanism whereby signaling molecules are released from the mitochondria is under intensive study. Possibilities include (i) transport by pore-forming proteins such as Bax (16), (ii) opening of the permeability transition pore (PTP) in the inner mitochondrial membrane leading to rupture of the outer mitochondrial membrane (17), or (iii) cooperation of the PTP and the voltage-dependent anion channel in the outer mitochondrial membrane (18).
In studies conducted with isolated mitochondria, a unifying element in the opening of PTP is the presence of high [Ca2+]. Here, we examine NMDA receptor-mediated neuronal apoptosis, which is known to involve high [Ca2+]i, and provide a model of apoptosis induced by mitochondrial dysfunction. This form of excitotoxic apoptosis is compared with staurosporine-induced apoptosis. The use of these two divergent inducers of apoptosis allows the elucidation of distinct steps leading to neuronal cell death.
Methods
Cerebrocortical Cell Cultures.
Cortical cultures were prepared as described previously (5). Briefly, after dissociation, cerebrocortical cells were plated on poly-l-lysine-coated dishes in DMEM with Ham's F-12 and horse serum at a ratio of 8:1:1. Cultures were incubated at 36.5°C in a humidified atmosphere composed of 5% CO2/95% air. In these cultures, approximately 20–30% of the cells were neurons, 70–80% astrocytes, and 5% microglia (5). Neurons could be reliably identified by morphology, as subsequently confirmed by immunolabeling with microtubule-associated protein-2 or NeuN.
Induction of Apoptosis.
Cells were exposed to 300 μM NMDA + 5 μM glycine for 20 min in Mg2+-free Earle's balanced salt solution (EBSS) ± bongkrekic acid (BA). Controls were incubated in Mg2+-free EBSS plus 5 μM glycine ± BA. After exposure to NMDA ± BA, cultures were rinsed with EBSS containing Mg2+ and replaced in culture medium. Staurosporine (0.5 μM, 0.1% DMSO) was added to the culture medium for 1 h or 18 h. Controls were exposed to 0.1% DMSO.
Assessment of Apoptosis.
For propidium iodide (PI) assays: cells were stained with 20 μg/ml PI after fixation with acetone and 4% paraformaldehyde/PBS. For SYTO-13 assays (19, 20): live cerebrocortical cells were stained with 50 nM SYTO-13 (Molecular Probes) at 37°C in Hepes-buffered medium (in mM, 137.6 NaCl/1 NaHCO3/0.34 Na2HPO4/5.36 KCl/0.44 KH2PO4/2.5 CaCl2/5 Hepes/22.2 glucose/1.3 MgCl2, pH 7.20). Apoptotic cells were distinguished morphologically by “rounded,” condensed nuclei, in contrast to larger, oval, healthy nuclei. For in situ end-labeling plus (ISEL+) detection of DNA strand breaks: cerebrocortical cells were fixed with 4% paraformaldehyde and permeabilized in 0.6% Triton X-100/PBS (21). Digoxigenin-labeled nucleotides were incorporated into DNA ends with terminal deoxynucleotidyltransferase (Roche Molecular Biochemicals). Polymerized label was visualized by immunohistochemistry. Total number of cells counted: 7,629 after NMDA exposure, 7,345 after BA/NMDA, and 4,746 after staurosporine.
Detection of Active Caspase-3 in Individual Neurons.
Cerebrocortical cells were fixed with acetone and 4% paraformaldehyde. After incubation in PBS/Tween 20 (0.05%) and 4% BSA, cells were incubated with 50 μM biotinylated-DEVD-CHO for 48 h (CHO indicating the 1-aldehyde analogue of the tetrapeptide). Next, cells were incubated with streptavidin-FITC for 3 h. FITC fluorescence intensity was examined by confocal microscopy [40× objective, excitation/emission (ex/em) 488/>515 nm].
Caspase Activity Assays in Cerebrocortical Lysates.
Caspase enzyme activity was monitored by cleavage of fluorescent peptide substrates (Enzyme Systems Products, Livermore, CA). The peptides, DEVD (caspase-3) and IETD (caspase-8), were selected on the basis of substrate specificity (22, 23). To measure caspase activity, cerebrocortical cells were washed in PBS and then lysed on ice in 100 mM Hepes (pH 7.40)/2 mM DTT/0.1% [(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS)/0.1% sucrose (for caspase-3), or 150 mM NaCl/20 mM Tris (pH 7.20)/1 mM DTT/1% Triton X-100 (for caspase-8). For the caspase-8 assays, the lysates were also incubated with an equal volume of reaction buffer: 20 mM KCl/20 mM Pipes (piperazine-N,N′-bis[2-ethanesulfonic acid]) (pH 7.40)/4 mM MgCl2/2 mM DTT. Supernatants were incubated with either DEVD-AFC (100 μM, 30 min), or IETD-AFC (50 μM, 60 min). AFC (7-amino-4-trifluoromethylcoumarin) fluorescence (ex/em 400/505 nm) was collected in a Fluoromax-2 fluorimeter. All measurements were corrected for protein concentration.
Estimation of Mitochondrial Membrane Potential (Δψm).
Tetramethylrhodamine methyl ester (TMRM; Molecular Probes) is a cationic dye that is accumulated by mitochondria according to the Nernst Equation. Uptake of TMRM allows a semiquantitative estimate of Δψm (24). Cerebrocortical cells were incubated in Hepes buffered medium containing 100 nM TMRM for 30 min. TMRM fluorescence from neuronal mitochondria was visualized by confocal microscopy (63× objective, 1.2 numerical aperture, ex 568 nm), and intensity of fluorescence was determined.
Bioenergetic Parameters.
Mitochondria were isolated from rat forebrains as described (25). To examine mitochondrial PTP, mitochondria were suspended in media containing 100 mM KCl, 5 mM K+-Tes (N-tris[hydroxymethyl]methyl-2-aminoethanesulfonic acid), 2 mM MgCl2, 5 mM KH2PO4, and 0.1 mg/ml BSA, pH 7.0. The same solution, with the addition of 0.5 mM EGTA, was used to measure oxygen consumption. Respiration was measured by using a Clark-type oxygen electrode (PP Systems). Swelling of mitochondria was assessed by light absorbance at 520 nm in a stirred suspension of mitochondria (26). The concentration of ATP was determined by firefly luciferase, as described (9).
Immunoprecipitation of Cyt c.
Lysis of cerebrocortical cells was performed at 4°C in 250 mM sucrose/25 mM Pipes (pH 7.5)/10 mM KCl/5 mM EGTA/1 mM DTT/100 μM digitonin, and a protease inhibitors mixture (Roche Molecular Biochemicals). Cyt c was immunoprecipitated by 2 μg/ml anti-Cyt c antibody (6H2.B4; PharMingen) plus protein A/G beads (Santa Cruz Biotechnology). The beads were pelleted, washed, and resuspended in SDS sample buffer. Electrophoresis of proteins was performed with Bis-Tris gels (NOVEX, San Diego) under reducing conditions. Proteins were transferred to nitrocellulose membranes and probed with anti-Cyt c antibody (7H8.2C12; PharMingen). Detection was performed with enhanced chemiluminescence (Amersham Pharmacia).
Statistics.
Data are given as mean ± SEM. Comparisons between two groups were assessed with a Student's t test. Comparisons between three or more sets of data were made with a one-way ANOVA followed by a post hoc Fisher's paired least significant difference (PLSD) of Scheffé multiple comparison of means. P values smaller than 0.05 were considered to be statistically significant.
Results
BA Protects from NMDA-Induced Neuronal Apoptosis.
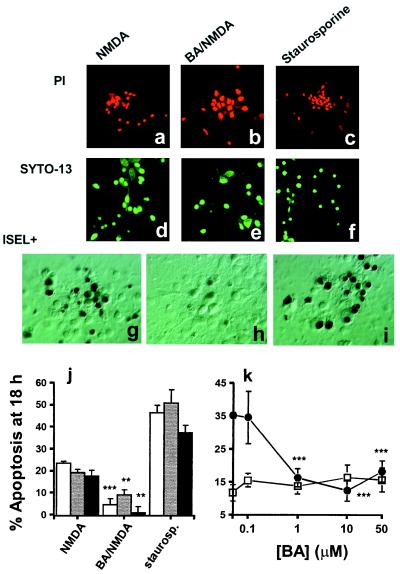
We have shown that cerebrocortical neurons in mixed cultures are susceptible to death by necrosis or apoptosis (5). In these cultures, necrotic cell death can be produced by a prolonged excitotoxic insult, e.g., 3 mM glutamate for 60 min, whereas neurons primarily undergo apoptosis after a shorter, 20-min exposure to 300 μM NMDA (5). In the present study, apoptosis was stimulated in a similar manner by incubating cerebrocortical cells with 300 μM NMDA plus 5 μM glycine in the absence of extracellular Mg2+ for 20 min. We assessed apoptosis after 18 h by examination for nuclear chromatin condensation. Such changes are characteristic of apoptosis, and can be monitored by visualizing the fluorescent DNA-binding probes PI in permeabilized cells (Fig. 1 a–c) or SYTO-13 in live cells (Fig. 1 d–f). We also used ISEL+ (21) to follow the appearance of DNA strand breaks (Fig. 1 g–i). These methods all showed that excessive NMDA receptor activation induced 20% of the cells to die by apoptosis (Fig. 1j). The number of necrotic cells under these conditions, indicated by PI staining of live cultures, was <1%. In addition, we found that a necrotic insult, 3 mM glutamate/5 μM glycine for 60 min, did not significantly increase the number of DNA strand breaks over control values (% ISEL+-positive nuclei at 18 h: 7.2 ± 2.7 vs. 8.5 ± 1.1%, respectively).
Figure 1.
Apoptosis in cerebrocortical cultures. Cultures were exposed to either 300 μM NMDA/5 μM glycine ± 1 μM BA, or 0.5 μM staurosporine. (a–c) Representative labeling of apoptotic cells with PI, (d–f) with SYTO-13, or (g–i) by ISEL+. (j) At 18 h post insult, the presence of cells with condensed nuclei was assessed by staining with either PI (open bars) or SYTO-13 (shaded bars). DNA strand breaks within individual cells were evaluated at 18 h by ISEL+ (filled bars). Values are corrected for the basal level of apoptosis in these cultures (11.7 ± 2.1%; mean ± SEM). Statistical significance of BA/NMDA compared with NMDA indicated by asterisks (**, P < 0.001; ***, P < 0.0001). (k) Protection produced by BA is concentration dependent. Cells treated with NMDA/glycine ± BA (●) were analyzed for apoptosis at 18 h using PI. Treatment of cultures with BA alone for 30 min did not result in an increase in apoptosis at 18 h (□). BA (0.1 μM) did not protect from NMDA-induced apoptosis (***, P < 0.001; n = 8).
We next investigated the effect of BA on NMDA receptor-induced apoptosis. BA has been shown to prevent apoptosis in cell-free extracts and in nonneuronal cells (17, 27). It has been proposed to prevent apoptosis by inhibiting the opening of the mitochondrial PTP (17). BA is a specific inhibitor of the adenine nucleotide translocator (ANT) (28), and several lines of investigation have suggested that the ANT is one component of the PTP complex (29, 30). We found that inhibition of the ANT with 1 μM BA for 10 min before and during a 20-min exposure to NMDA/glycine reduced neuronal apoptosis by several criteria (Fig. 1 b, e, h, and j, mean level of apoptosis 4.8% above control). The protection from NMDA-induced apoptosis afforded by BA was concentration-dependent (Fig. 1k), with maximal efficacy at concentrations of 1 μM and above. ANT inhibition causes inactivation of oxidative phosphorylation and other reactions requiring adenine nucleotides. Nonetheless, exposure to BA alone (0.1–50 μM for 30 min) did not induce any significant increase in apoptosis at 18 h (Fig. 1k).
Cyclosporine A (CsA, 10 μM), an alternative inhibitor of the PTP, was also capable of decreasing the extent of NMDA receptor-mediated apoptosis (% apoptosis 5.2 ± 3.8 above control, data not shown). However, although CsA has demonstrated neuroprotective properties (31), it is known to have multiple sites of action (32), and thus its neuroprotection cannot be attributed specifically to inhibition of the PTP.
BA Does Not Prevent Staurosporine-Induced Apoptosis.
Staurosporine initiates apoptosis in many different cell types, including neurons (33, 34). Although its precise modus operandi remains unknown, staurosporine was used here as a positive control. When added to cultures, 0.5 μM staurosporine stimulated the death of up to 50% of the cells (Fig. 1j). This greater effect than NMDA reflects the ability of staurosporine to induce apoptosis in all cell types, whereas NMDA only affects neurons because only this cell type manifests NMDA receptors in these cultures. In contrast to its effects on NMDA-induced apoptosis, 1 μM BA did not reduce the extent of apoptosis induced by a 1-h exposure to staurosporine (% apoptosis 55.9 ± 3.3 above control, data not shown).
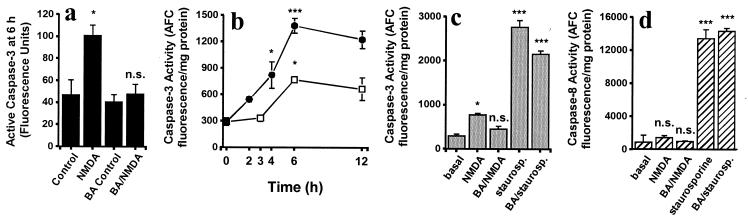
BA Neuroprotection Is Accompanied by a Decrease in Caspase-3 Activity.
Activation of caspases can be investigated before the relatively late morphologic changes of apoptosis. Caspase-3 is recognized as an effector caspase, and its activation in NMDA receptor-induced apoptosis represents a commitment to the subsequent death of neurons (35, 36). Here, we monitored caspase-3 activity in two ways: in individual cells with biotinylated DEVD, and in cell lysates using a fluorogenic substrate. Within 6 h of NMDA exposure, conformationally active caspase-3 was apparent in individual neurons (Fig. 2a). At this time, maximal caspase-3-like activity was detected in cell lysates (Fig. 2b). Incubation with staurosporine initiated a significant increase in caspase-3 activity within 2 h, and by 6 h produced more caspase-3 activity than that seen in NMDA-treated cultures (Fig. 2b). In lysates made from cultures treated with BA during NMDA receptor activation, caspase-3 activity at 6 h was significantly lower than that seen after NMDA alone (Fig. 2c). Furthermore, after NMDA/BA exposure, conformationally active caspase-3 was not above basal in individual neurons (Fig. 2a). Although BA appeared to decrease staurosporine-induced activation of caspase-3 to some degree, the effect did not reach statistical significance (Fig. 2c).
Figure 2.
Determination of caspase activity. (a) Quantification of conformationally active caspase-3 in individual neurons (biotinylated-DEVD). At 6 h, the amount of active caspase-3 after BA/NMDA was not significantly different (n.s.) from the basal level, but was statistically less than that of NMDA (*, P < 0.05). (b and c) Detection of caspase-3 activity in cerebrocortical cell lysates by cleavage of fluorogenic substrates. (b) NMDA-induced activation (□) of caspase-3 peaked after 6 h (*, P < 0.05 from basal; n = 12). Staurosporine-treated samples (●) displayed a large increase in caspase-3 at 6 h (***, P < 0.001; n = 12). (c) BA prevented NMDA-induced caspase-3 activity at 6 h. The BA/NMDA value was not significantly greater than the basal value but was significantly different from NMDA (*, P < 0.05; n = 12). Staurosporine (staurosp.) values were greater compared with basal (***, P < 0.001; n = 6), but were not statistically decreased by BA. (d) Caspase-8 activity 6 h after exposure to NMDA or staurosporine. Under our conditions, NMDA did not significantly stimulate activation of caspase-8 (n = 8), whereas staurosporine markedly increased caspase-8 (***, P < 0.001: n = 3).
Staurosporine, but Not NMDA, Initiates Activation of Caspase-8.
Caspase-8 is an upstream component of receptor signaling-induced apoptosis. Following autoactivation, caspase-8, cleaves both Bid and caspase-3 (15, 37). We found that after 6 h of exposure to staurosporine, caspase-8 enzyme activity increased 20-fold above basal in cerebrocortical cell lysates (Fig. 2d). In contrast, NMDA receptor activation (±BA) was not associated with a significant increase in caspase-8 activity, and BA did not reduce staurosporine-induced activation of caspase-8.
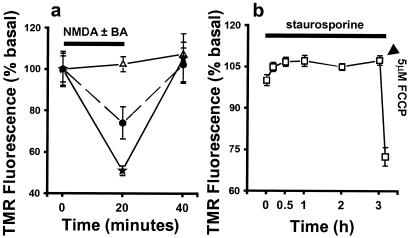
BA Prevents NMDA-Induced Δψm Depolarization.
One consequence of NMDA receptor activation is a large Ca2+ influx into neurons, resulting in increased [Ca2+]i to concentrations greater than 1 μM (7, 9, 10, 36). It is known that mitochondrial Ca2+ accumulation occurs at [Ca2+]i = 0.3–0.5 μM, and that this can mediate Δψm depolarization (38). For that reason, we made an estimation of Δψm by assessing changes in the fluorescence intensity of TMRM accumulated in mitochondria within individual cerebrocortical neurons. At low, nonquenching concentrations of dye, depolarization of Δψm is accompanied by both a decrease in fluorescence and a loss of punctate localization of the dye as it transfers from the mitochondria into the cytoplasm. NMDA receptor activation in cerebrocortical neurons produced a 26% decrease in fluorescence intensity (n = 80 neurons; Fig. 3a). The depolarization of Δψm induced by NMDA receptor activation was not complete, as demonstrated by a greater decrease in fluorescence intensity produced by the protonophore carbonylcyanide p-trifluoromethoxyphenylhydrazone (FCCP) (Fig. 3a). After removal of NMDA, TMRM fluorescence returned to basal levels, indicating a repolarization of Δψm. In the absence of external Ca2+, NMDA did not result in a decrease in TMRM fluorescence (data not shown), confirming that the TMRM signal was because of the Δψm depolarization associated with Ca2+ uptake and not to NMDA-induced plasma membrane depolarization. Incubation in 1 μM BA completely abrogated the NMDA-induced decrease in Δψm (n = 60 neurons; Fig. 3a). However, BA did not prevent FCCP-induced Δψm depolarization (data not shown). In contrast to these events, exposure to staurosporine for up to 3 h had no effect on Δψm (Fig. 3b). Furthermore, after 3 h of staurosporine exposure, the mitochondria were still responsive to depolarization by FCCP (Fig. 3b).
Figure 3.
BA prevents NMDA-induced depolarization of Δψm in cerebrocortical neurons. (a) NMDA receptor activation induced a partial depolarization of Δψm (●). Incubation with 1 μM BA (▵) prevented the NMDA receptor-induced reduction in Δψm. Depolarization induced by 1 μM FCCP (★) further decreased TMRM fluorescence intensity. (b) Staurosporine did not depolarize mitochondria for at least 3 h, at which time Δψm was still sensitive to FCCP.
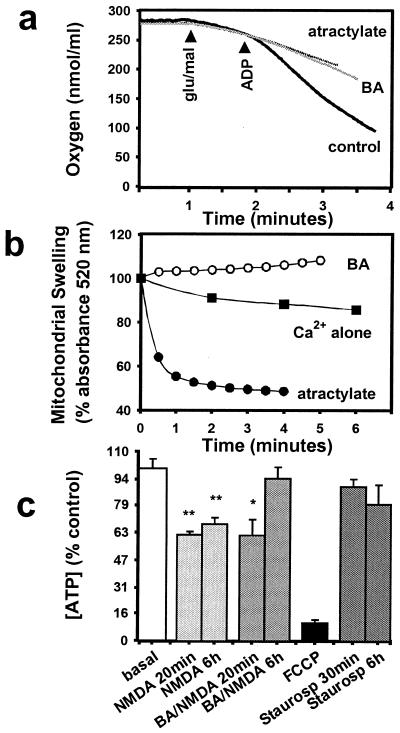
The Effect of BA on Bioenergetics.
We examined the effect of BA on isolated brain mitochondria. It is known that both BA and atractylate inhibit ATP/ADP transport by stabilizing the ANT in inactive but physically opposite conformations (28). Consistent with this mechanism, addition of either BA or atractylate to a suspension of isolated brain mitochondria prevented the stimulation of oxygen consumption elicited by addition of ADP (Fig. 4a). A greater consumption of O2 with the addition of ADP marks the stimulation of respiration. However, BA inhibits, whereas atractylate opens the PTP (26). Opening of the PTP results in movement of small solutes across the inner mitochondrial membrane, with consequent osmotic swelling of the mitochondria. Swelling is readily followed by measuring light scattering in suspensions of mitochondria; swelling correlates with a decrease in light scattering. We found that under de-energized conditions, produced by incubation of brain mitochondria for 4 min in FCCP, phosphoenolpyruvate, and the calcium ionophore A23187 (26), further addition of 500 nM Ca2+ induced a small but significant swelling of brain mitochondria (Fig. 4b; P < 0.05 comparing values at time t = 0 min vs. t = 6 min). However, no swelling was observed if 5 μM BA was added to the suspension 15 s before Ca2+ (Fig. 4b; P < 0.05 compared with Ca2+ alone at t = 6 min). Addition of atractylate before Ca2+ exposure precipitated extensive swelling of the mitochondria (Fig. 4b; P < 0.0001 compared with Ca2+ alone at t = 4 min).
Figure 4.
Effect of BA on isolated brain mitochondria supported by glutamate/malate oxidation. (a) BA (5 μM) and atractylate (20 μM) both inhibited stimulation of respiration on addition of 0.5 mM ADP. (b) Mitochondrial swelling induced by addition of 0.5 μM Ca2+ to de-energized mitochondria at time t = 0 was enhanced in the presence of 20 μM atractylate (●) but prevented by 5 μM BA (○). (c) The concentration of cellular ATP in cerebrocortical cells fell within 20 min of exposure to NMDA, but recovered by 6 h in the presence of BA. Staurosporine was not associated with a fall in ATP concentration (*, P < 0.05; **, P < 0.005; n = 3).
NMDA receptor activation, and Ca2+-loading of neuronal mitochondria, leads to a decline in cellular energy (9, 19). Activation of NMDA receptors for 20 min reduced the amount of ATP in cerebrocortical cells (Fig. 4c). Incubation with 1 μM BA did not prevent the initial NMDA-mediated fall in ATP, but it did enhance the recovery of ATP levels 6 h after NMDA exposure. BA alone did not affect ATP levels (96.4 ± 11.0% of basal value at 6 h, n = 3). In contrast, a 20-min incubation with 5 μM FCCP dramatically reduced ATP to 10% of the basal level (Fig. 4c). This large reduction in ATP concentration is caused by the mitochondrial ATP synthase running in reverse, hydrolyzing ATP (9). During a similar time period, 0.5 μM staurosporine did not significantly affect ATP levels (Fig. 4c).
Exit of Cyt c from Mitochondria.
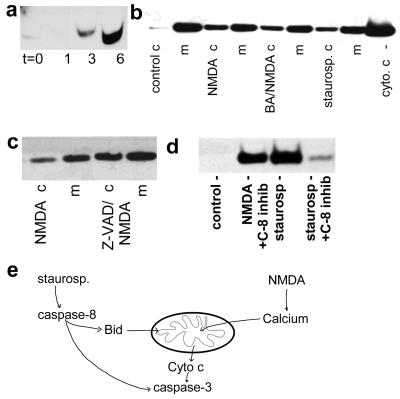
The release of Cyt c from mitochondria is considered a decisive event leading to apoptosis (11, 14). By immunoprecipitation of Cyt c from cytoplasmic fractions, we detected Cyt c as early as 3 h following NMDA receptor activation (Fig. 5a). NMDA receptor stimulation induced only a partial release of Cyt c, for at 6 h substantial amounts remained in the mitochondrial or membrane-associated fraction (Fig. 5b). The presence of BA during NMDA receptor activation did not prevent the movement of Cyt c into the cytoplasm (Fig. 5b). Because BA prevented both caspase-3 activation and NMDA-induced apoptosis, this result indicates that, in neurons, the presence of Cyt c in the cytoplasm is not sufficient for triggering apoptosis.
Figure 5.
Release of Cyt c. (a) Western blot of Cyt c reveals appearance of Cyt c in the cytoplasm of cerebrocortical cells 3 and 6 h after NMDA receptor activation. (b) Cyt c was detected not only in membrane (m) but also in cytosolic (c) fractions 6 h after exposure to NMDA, NMDA plus BA, or staurosporine; however, under control conditions (Left) cytoplasmic Cyt c remained virtually undetectable. Under all conditions, most of the Cyt c remained in the membrane fraction. (c) Inhibition of caspases with 50 μM z-VAD-fmk (z = benzyloxycarbonyl; fmk = fluoromethyl ketone) during NMDA receptor activation did not prevent release of Cyt c. (d) Inhibition of caspase-8 with 20 μM z-IETD-fmk blocked staurosporine-induced release of Cyt c. (e) Model of staurosporine and NMDA activation of caspases and Cyt c release.
Staurosporine (0.5 μM for 6 h) was also associated with release of Cyt c from mitochondria (Fig. 5b). Because staurosporine did not induce Δψm depolarization, this indicates that, in cerebrocortical cells, release of Cyt c from mitochondria does not require Δψm depolarization.
Inhibition with a pan-caspase antagonist before and during NMDA receptor activation was ineffective in preventing release of Cyt c (Fig. 5c). This confirms that release of Cyt c is upstream of caspase activation in this paradigm. In contrast, specific inhibition of caspase-8 (z-IETD-fmk, 20 μM) reduced the extent of Cyt c release by staurosporine (Fig. 5d). This finding further indicates that staurosporine and excitotoxic (NMDA) insults use distinct signaling pathways to effect apoptosis.
Discussion
In the present study, we have analyzed key neuronal events in the signaling from excessive NMDA receptor stimulation to apoptosis, and contrast this to the apoptotic pathway induced by staurosporine. Here, we demonstrate that NMDA receptor-induced apoptosis is associated with an increase in caspase-3 activity, Δψm depolarization, Cyt c release, and ATP loss. BA blocks NMDA receptor-mediated apoptosis but does not prevent Cyt c release. Finally, we show that BA does not prevent staurosporine-induced apoptosis in cerebrocortical cells, nor is staurosporine associated with either Δψm depolarization or ATP depletion. Furthermore, unlike the pathway invoked by NMDA, apoptosis induced by staurosporine is associated with an increase in caspase-8 activity.
Mitochondrial Dysfunction Mediates NMDA Receptor-Induced Apoptosis.
We asked the question whether NMDA receptor-mediated apoptosis of cerebrocortical neurons involves mitochondrial dysfunction. It is known that BA binds to the ANT, which is thought to block opening of the PTP. Therefore, protection afforded by BA in the present study implicates the ANT and the PTP in signaling to NMDA-induced apoptosis. Opening of the PTP has drastic consequences for the individual mitochondrion, including a complete collapse in Δψm, hydrolysis of ATP, and release of mitochondrially accumulated Ca2+ (40). Several findings in this study suggest that opening of the PTP is not the initial, widespread, or master switch in apoptosis that it has been posited to be. First, NMDA produced only partial and transient depolarization of Δψm rather than complete collapse. This finding can be explained by permeability transition, which is an all-or-none event, of only a small fraction of mitochondria in a given neuron. Second, the initial fall in ATP concentration observed 20 min after NMDA exposure was not prevented by BA and was less than that induced by FCCP (Fig. 4c), suggesting that mitochondria are not involved in this initial de-energization. This loss of ATP after NMDA exposure could, however, be important for subsequent opening of the PTP. Third, that BA blocked NMDA-induced caspase-3 activation and apoptosis but not the release of Cyt c indicates that the ANT/PTP does not mediate Cyt c release and, additionally, that Cyt c is not sufficient for neuronal apoptosis.
Why then is BA protective? A possible clue is provided by Δψm: In the presence of BA, the mitochondria are able to withstand an NMDA-induced Ca2+ load without Δψm depolarization (Fig. 3a). Similarly, in neural cells, the overexpression of Bcl2 allows mitochondria in situ to accumulate Ca2+ without affecting Δψm (42). Furthermore, Bcl2 has been shown to directly interact with the ANT and regulate the exchange of ATP for ADP (43), indicating a possible similar mechanism for BA. We have observed that although the initial NMDA-induced decline in ATP concentration is still evident in the presence of BA, at a later time the ATP concentration recovers (Fig. 4c). It is possible that modulation of availability of adenine nucleotides by either Bcl2 or BA may influence apoptosis. Along these lines, it is already known that some ATP is required for apoptosis (19, 44) and that dATP along with Cyt c are cofactors for caspase activation (45).
Release of Cyt c Is Not Sufficient for Neuronal Cell Death.
Release of Cyt c from mitochondria has received much attention as a commitment to apoptosis (11, 12, 14, 18). The present study shows that release of Cyt c from in situ mitochondria is not sufficient for neuronal apoptosis. Similar observations have been made for nonneuronal cells (47), and microinjection of Cyt c into sympathetic neurons does not lead to death in the absence of additional stimuli (46). We show that although BA can prevent the activation of caspase-3, Cyt c is still released into the cytosol (Fig. 5b). Therefore, Cyt c must require another decisive factor(s) to initiate the caspase cascade. In addition to dATP (discussed above), Apaf-1 and procaspase-9 are other known candidates from work in nonneuronal cells (48).
Staurosporine-Induced, but Not NMDA-Induced, Apoptosis Occurs in the Absence of Mitochondrial Depolarization.
Recent studies have focused on Δψm depolarization as a primary event in apoptosis (13, 17, 27). The assumption has been that because Δψm depolarization accompanies opening of the PTP, Δψm depolarization can therefore serve as a marker of apoptosis. However, although mitochondrial Δψm depolarization does accompany opening of the PTP, it also occurs reversibly and independently of PT, for example in the presence of FCCP (Fig. 3a). Furthermore, release of death effectors can occur while Δψm remains high, suggesting release by mechanisms other than PTP (11, 12, 14, 39, 49). We show that NMDA receptor-mediated apoptosis is associated with partial Δψm depolarization (Fig. 3a). In contrast, staurosporine is not associated with Δψm depolarization, indicating that Δψm depolarization per se is not an essential step in apoptosis. Staurosporine-induced apoptosis is accompanied by release of Cyt c and caspase-3 activation. However, BA did not protect against staurosporine-induced apoptosis. This, along with the absence of an effect of staurosporine on Δψm or ATP concentration, suggests that staurosporine activates caspases without directly disrupting mitochondrial energy production. Furthermore, staurosporine, but not NMDA-mediated apoptosis, is associated with an increase in caspase-8 activity (Fig. 2d), and caspase-8 inhibition greatly reduces staurosporine-induced release of Cyt c (Fig. 5d). In nonneuronal cells (15, 36), caspase-3 can be activated by caspase-8, or by means of Bid cleavage and subsequent release of Cyt c. As shown here, these pathways also appear to be present in neurons. This leads us to the important point of view that staurosporine, although an effective inducer of apoptosis, does not reproduce the sequence of events that occurs in neurons dying from excitotoxic-related events (Fig. 5e). Moreover, excessive NMDA receptor stimulation, unlike staurosporine, can disrupt neuronal mitochondrial function, leading to activation of apoptotic pathways.
Overall, our study questions the significance of the mitochondrial PTP and release of Cyt c as the sole mechanism for induction of neuronal apoptosis, and indicates that further investigation is needed to elucidate the role of the various components of the PTP in different cell death pathways.
Acknowledgments
We thank Danielle D'Emilia for expert technical assistance and Gwenn Garden for helpful discussions. BA was a kind gift from Prof. J. A. Duine (Delft, The Netherlands). This work was supported in part by National Institutes of Health Grants from the National Eye Institute (R01 EY05477 and R01 EY09024) and the National Institute of Child Health and Human Development (P01 HD29587) (to S.A.L.), and by a Wellcome Trust Research Traveling Fellowship (to S.L.B.).
Abbreviations
- BA
bongkrekic acid
- Cyt c
cytochrome c
- NMDA
N-methyl-d-aspartate
- Δψm
mitochondrial membrane potential
- PTP
permeability transition pore
- PI
propidium iodide
- TMRM
tetramethylrhodamine methyl ester
- ANT
adenine nucleotide translocator
- [Ca2+]i
intracellular free calcium concentration
- ISEL+
in situ end-labeling plus
- ex/em
excitation/emission
- FCCP
carbonylcyanide p-trifluoromethoxyphenylhydrazone
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.100121097.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.100121097
References
- 1.Loo D T, Copani A, Pike C J, Whittemore E R, Walencewicz A J, Cotman C W. Proc Natl Acad Sci USA. 1993;90:7951–7955. doi: 10.1073/pnas.90.17.7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Portera-Cailliau C, Hedreen J C, Price D L, Koliatsos V E. J Neurosci. 1995;15:3775–3787. doi: 10.1523/JNEUROSCI.15-05-03775.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lipton S A. Brain Pathol. 1996;6:507–517. doi: 10.1111/j.1750-3639.1996.tb00879.x. [DOI] [PubMed] [Google Scholar]
- 4.Burke R E, Kholodilov G. Ann Neurol. 1998;44:S126–S133. doi: 10.1002/ana.410440719. [DOI] [PubMed] [Google Scholar]
- 5.Bonfoco E, Krainc D, Ankarcrona M, Nicotera P, Lipton S A. Proc Natl Acad Sci USA. 1995;92:7162–7166. doi: 10.1073/pnas.92.16.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ayata C, Ayata G, Hara H, Matthews R T, Beal M F, Ferrante R J, Endres M, Kim A, Christie R H, Waeber C, et al. J Neurosci. 1997;17:6908–6917. doi: 10.1523/JNEUROSCI.17-18-06908.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dubinsky J M. J Neurosci. 1993;13:623–631. doi: 10.1523/JNEUROSCI.13-02-00623.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herman B, Gores G J, Nieminen A L, Kawanishi T, Harman A, Lemasters J J. Toxicology. 1990;21:127–148. doi: 10.3109/10408449009089876. [DOI] [PubMed] [Google Scholar]
- 9.Budd S L, Nicholls D G. J Neurochem. 1996;67:2281–2291. doi: 10.1046/j.1471-4159.1996.67062282.x. [DOI] [PubMed] [Google Scholar]
- 10.White R J, Reynolds I J. J Physiol (London) 1997;498:31–47. doi: 10.1113/jphysiol.1997.sp021839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kluck R M, Bossy-Wetzel E, Green D R, Newmeyer D D. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 12.Yang J, Liu X, Bhalla K, Kim C N, Ibrado A M, Cai J, Peng T I, Jones D P, Wang X. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 13.Susin S A, Zamzami N, Castedo M, Hirsch T, Marchetti P, Macho A, Daugas E, Geuskens M, Kroemer G. J Exp Med. 1996;184:1331–1341. doi: 10.1084/jem.184.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bossy-Wetzel E, Green D R. J Biol Chem. 1999;274:17484–17490. doi: 10.1074/jbc.274.25.17484. [DOI] [PubMed] [Google Scholar]
- 15.Li H, Zhu H, Xu C-J, Yuan J. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 16.Marzo I, Brenner C, Zamzami N, Jurgensmeier J M, Susin S A, Vieira H L, Revost M C, Xie Z, Matsuyama S, Reed J C, et al. Science. 1998;281:2027–2031. doi: 10.1126/science.281.5385.2027. [DOI] [PubMed] [Google Scholar]
- 17.Zamzami N, Susin S A, Marchetti P, Hirsch T, Gomez-Monterrey I, Castedo M, Kroemer G. J Exp Med. 1996;183:1533–1544. doi: 10.1084/jem.183.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimizu S, Narita M, Tsujimoto Y. Nature (London) 1999;399:483–487. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- 19.Ankarcrona M, Dypbukt J M, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton S A, Nicotera P. Neuron. 1995;15:1961–1973. doi: 10.1016/0896-6273(95)90186-8. [DOI] [PubMed] [Google Scholar]
- 20.Poot M, Gibson L L, Singer V L. Cytometry. 1997;27:358–364. [PubMed] [Google Scholar]
- 21.Blaschke A J, Staley K, Chun J. Development (Cambridge, UK) 1996;122:1165–1174. doi: 10.1242/dev.122.4.1165. [DOI] [PubMed] [Google Scholar]
- 22.Thornberry N A, Rano T A, Peterson E P, Rasper D M, Timkey T, Garcia-Calvo M, Houtzager V M, Nordstrom P A, Roy S, Vaillancourt J P, et al. Proc Natl Acad Sci USA. 1997;272:17907–17911. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- 23.Talanian R V, Quinlan C, Trautz S, Hackett M C, Mankovich J A, Banach D, Ghayur T, Brady K D, Wong W W. J Biol Chem. 1997;272:9677–9682. doi: 10.1074/jbc.272.15.9677. [DOI] [PubMed] [Google Scholar]
- 24.Ehrenberg B, Montana V, Wei M-D, Wuskell J P, Loew L M. Biophys J. 1988;53:785–794. doi: 10.1016/S0006-3495(88)83158-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai J C K, Clark J B. Methods Enzymol. 1979;55:51–59. doi: 10.1016/0076-6879(79)55008-3. [DOI] [PubMed] [Google Scholar]
- 26.Hunter D R, Haworth R A. Arch Biochem Biophys. 1979;195:453–459. doi: 10.1016/0003-9861(79)90371-0. [DOI] [PubMed] [Google Scholar]
- 27.Marchetti P, Hirsch T, Zamzami N, Castedo M, Decaudin D, Susin S A, Masse B, Kroemer G. J Immunol. 1996;157:4830–4836. [PubMed] [Google Scholar]
- 28.Klingenberg M, Grebe K, Heldt H W. Biochem Biophys Res Commun. 1970;39:344–351. doi: 10.1016/0006-291x(70)90582-6. [DOI] [PubMed] [Google Scholar]
- 29.Beutner G, Ruck A, Riede B, Welte W, Brdiczka D. FEBS Lett. 1996;396:189–195. doi: 10.1016/0014-5793(96)01092-7. [DOI] [PubMed] [Google Scholar]
- 30.Halestrap A P, Woodfield K-Y, Connern C P. J Biol Chem. 1997;272:3346–3354. doi: 10.1074/jbc.272.6.3346. [DOI] [PubMed] [Google Scholar]
- 31.Schinder A F, Olson E C, Spitzer N C, Montal M. J Neurosci. 1996;16:6125–6133. doi: 10.1523/JNEUROSCI.16-19-06125.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ankarcrona M, Dypbukt J M, Orrenius S, Nicotera P. FEBS Lett. 1996;394:321–324. doi: 10.1016/0014-5793(96)00959-3. [DOI] [PubMed] [Google Scholar]
- 33.Koh J-Y, Wie M B, Gwag B J, Sensi S L, Canzoniero L M T, Demaro J, Csernansky C, Choi D W. Exp Neurol. 1995;135:153–159. doi: 10.1006/exnr.1995.1074. [DOI] [PubMed] [Google Scholar]
- 34.Krohn A J, Preis E, Prehn J H M. J Neurosci. 1998;18:8186–8197. doi: 10.1523/JNEUROSCI.18-20-08186.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thornberry N A, Lazebnik Y. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 36.Tenneti L, D'Emilia D M, Troy C M, Lipton S A. J Neurochem. 1998;71:946–959. doi: 10.1046/j.1471-4159.1998.71030946.x. [DOI] [PubMed] [Google Scholar]
- 37.Stennicke H R, Jurgensmeier J M, Shin J M, Deveraux Q, Wolf B B, Yang X, Zhou Q, Ellerby H M, Ellerby L M, Bredesen D E, et al. J Biol Chem. 1998;273:27084–27090. doi: 10.1074/jbc.273.42.27084. [DOI] [PubMed] [Google Scholar]
- 38.Åkerman K. Biochim Biophys Acta. 1978;502:359–366. doi: 10.1016/0005-2728(78)90056-7. [DOI] [PubMed] [Google Scholar]
- 39.Bossy-Wetzel E, Newmeyer D D, Green D R. EMBO J. 1998;17:37–49. doi: 10.1093/emboj/17.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murphy A N, Bredesen D E, Cortopassi G, Wang E, Fiskum G. Proc Natl Acad Sci USA. 1996;93:9893–9898. doi: 10.1073/pnas.93.18.9893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andreyev A Y, Fahy B, Fiskum G. FEBS Lett. 1998;439:373–376. doi: 10.1016/s0014-5793(98)01394-5. [DOI] [PubMed] [Google Scholar]
- 42.Murphy A N, Fiskum G, Beal M F. J Cereb Blood Flow Metab. 1999;19:231–245. doi: 10.1097/00004647-199903000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Heiden M G, Chandel N S, Schumacker P T, Thompson C B. Mol Cell. 1999;3:159–167. doi: 10.1016/s1097-2765(00)80307-x. [DOI] [PubMed] [Google Scholar]
- 44.Leist M, Single B, Castoldi A F, Kuhnle S, Nicotera P. J Exp Med. 1997;185:1481–1486. doi: 10.1084/jem.185.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li P, Nijhawan D, Budihardjo I, Srinivasula S M, Ahmed M, Alnemri E S, Wang X. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 46.Deshmukh M, Johnson E M., Jr Neuron. 1998;21:695–705. doi: 10.1016/s0896-6273(00)80587-5. [DOI] [PubMed] [Google Scholar]
- 47.Rossé T, Olivier R, Monney L, Rager M, Conus S, Fellay I, Jansen B, Borner C. Nature (London) 1998;391:496–499. doi: 10.1038/35160. [DOI] [PubMed] [Google Scholar]
- 48.Liu X, Kim C N, Yang J, Jemmerson R, Wang X. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 49.Krohn A J, Wahlbrink T, Prehn J H M. J Neurosci. 1999;19:7394–7404. doi: 10.1523/JNEUROSCI.19-17-07394.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]