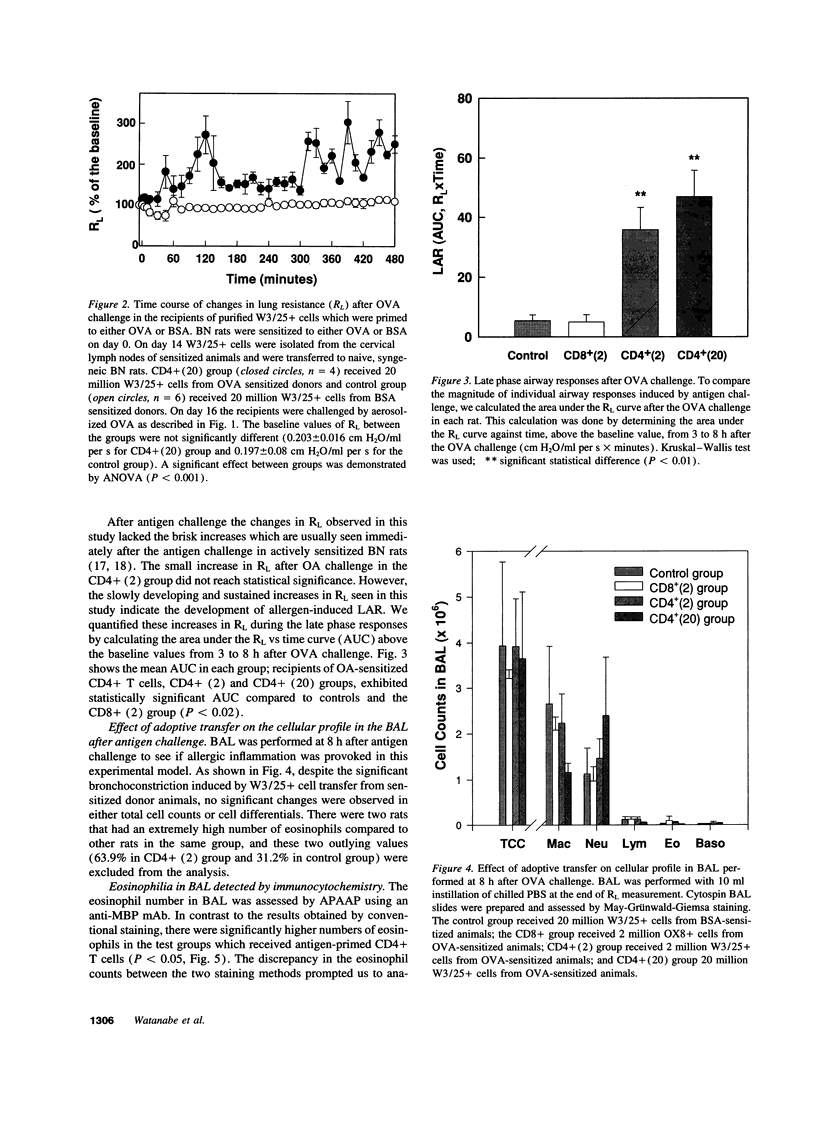
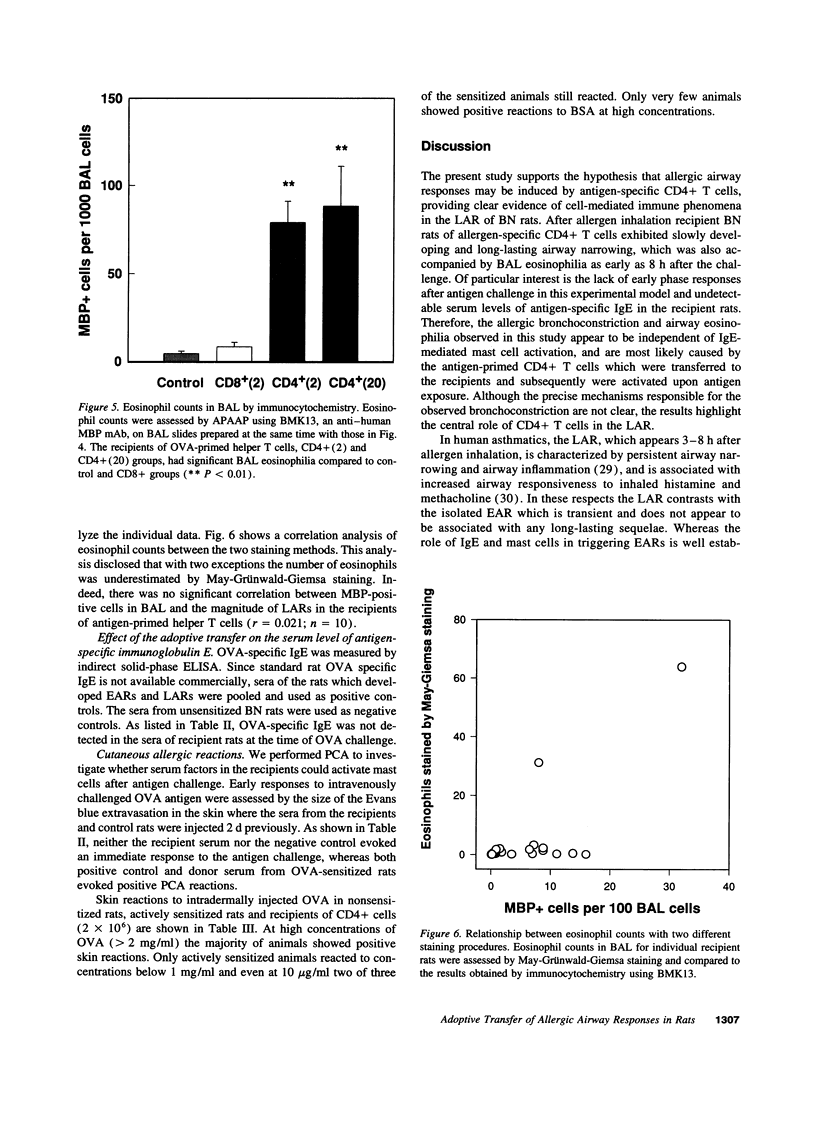
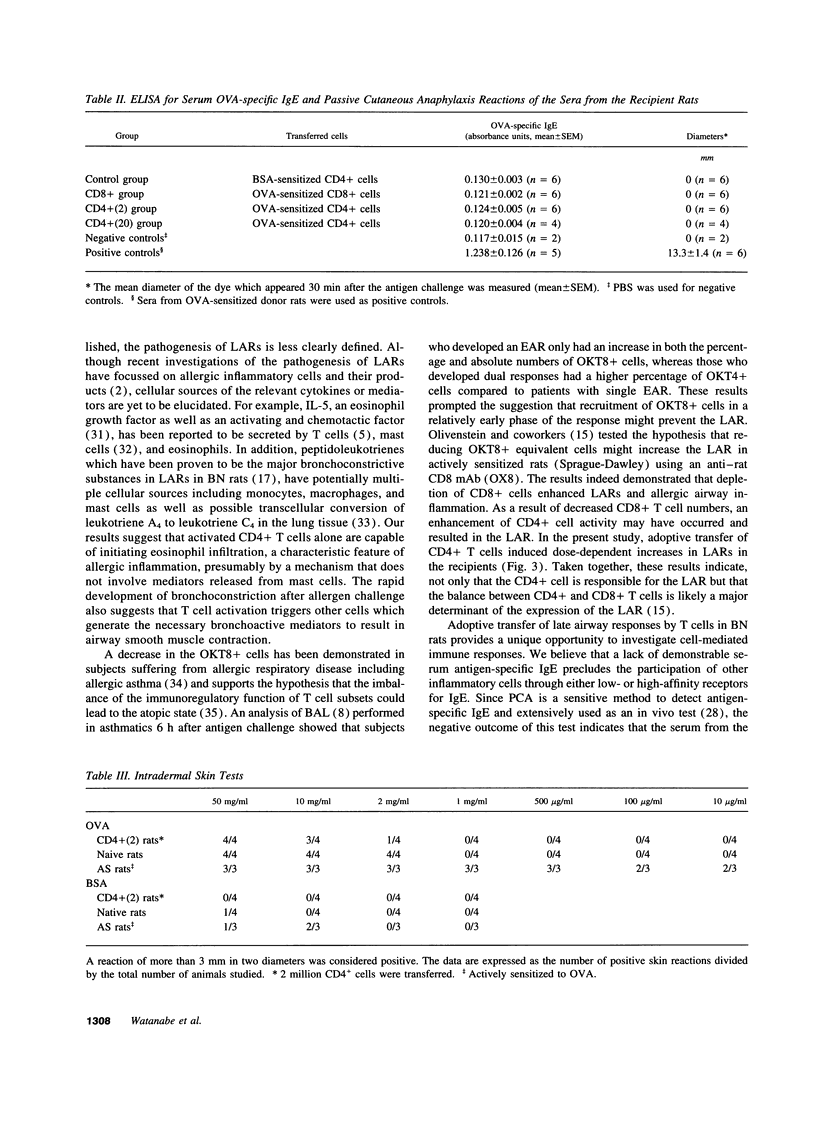
Abstract
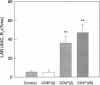
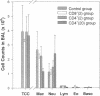
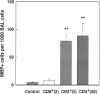
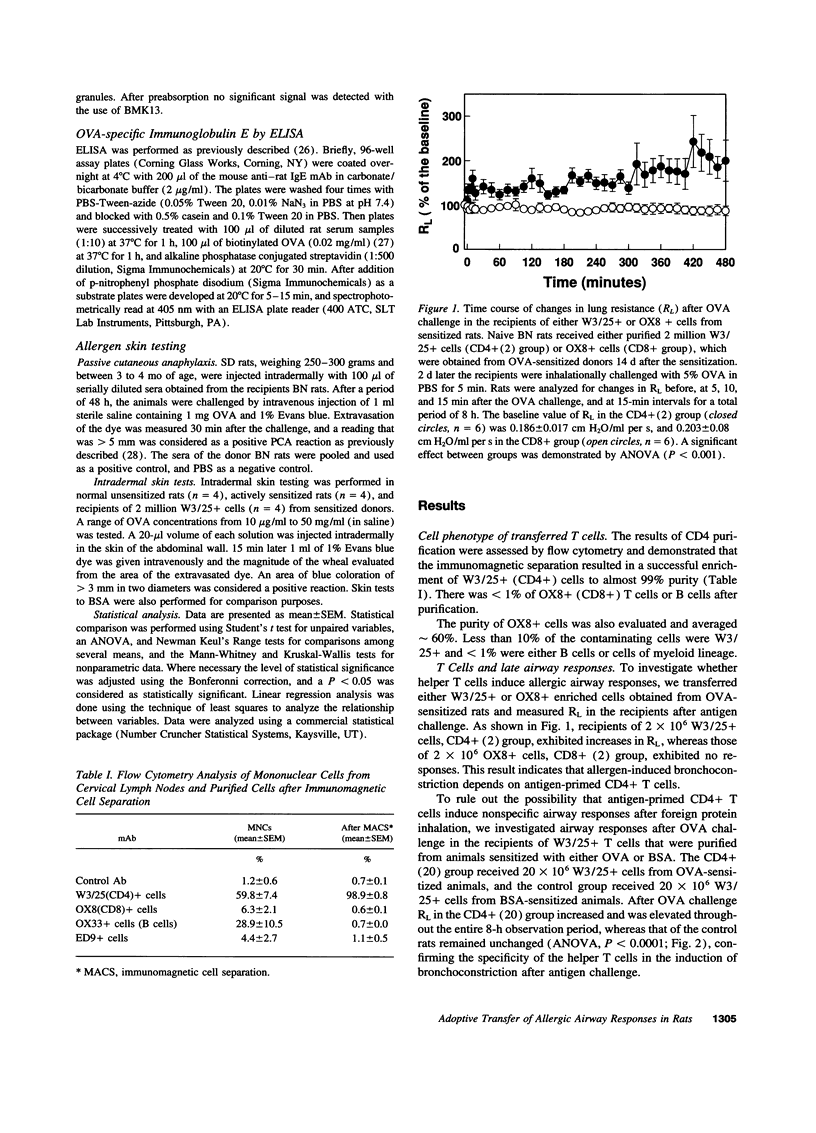
Activated CD4+ helper T cells have been demonstrated in asthmatic airways and postulated to play a central role in eliciting allergic inflammation; direct evidence of their involvement seems to be lacking. We hypothesized that CD4+ T cells have the potential to induce allergic responses to antigen challenge, and tested this hypothesis in a model of allergic bronchoconstriction, the Brown Norway rat, using the approach of adoptive transfer. Animals were actively sensitized to either ovalbumin (OVA) or BSA and were used as donors of T cells. W3/25(CD4)+ or OX8(CD8)+ T cells were isolated from the cervical lymph nodes of sensitized donors and transferred to naive BN rats. 2 d after adoptive transfer recipient rats were challenged by OVA inhalation, and changes in lung resistance (RL), bronchoalveolar lavage (BAL) cells, and serum levels of antigen-specific IgE were studied. After OVA challenge recipients of OVA-primed W3/25+ T cells exhibited sustained increases in RL throughout the entire 8-h observation period and had significant bronchoalveolar lavage eosinophilia, which was detected by immunocytochemistry using an antimajor basic protein mAb. Recipients of BSA-primed W3/25+ T cells or OVA-primed OX8+ T cells failed to respond to inhaled OVA. OVA-specific immunoglobulin E was undetectable by ELISA or skin testing in any of the recipient rats after adoptive transfer. In conclusion, antigen-induced airway bronchoconstriction and eosinophilia were successfully transferred by antigen-specific W3/25+ T cells in Brown Norway rats. These responses were dependent on antigen-primed W3/25+ T cells and appeared to be independent of IgE-mediated mast cell activation. This study provides clear evidence for T cell mediated immune mechanisms in allergic airway responses in this experimental model.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azzawi M., Bradley B., Jeffery P. K., Frew A. J., Wardlaw A. J., Knowles G., Assoufi B., Collins J. V., Durham S., Kay A. B. Identification of activated T lymphocytes and eosinophils in bronchial biopsies in stable atopic asthma. Am Rev Respir Dis. 1990 Dec;142(6 Pt 1):1407–1413. doi: 10.1164/ajrccm/142.6_Pt_1.1407. [DOI] [PubMed] [Google Scholar]
- Berman J. S., Beer D. J., Theodore A. C., Kornfeld H., Bernardo J., Center D. M. Lymphocyte recruitment to the lung. Am Rev Respir Dis. 1990 Jul;142(1):238–257. doi: 10.1164/ajrccm/142.1.238. [DOI] [PubMed] [Google Scholar]
- Booij-Noord H., de Vries K., Sluiter H. J., Orie N. G. Late bronchial obstructive reaction to experimental inhalation of house dust extract. Clin Allergy. 1972 Mar;2(1):43–61. doi: 10.1111/j.1365-2222.1972.tb01267.x. [DOI] [PubMed] [Google Scholar]
- Bradding P., Roberts J. A., Britten K. M., Montefort S., Djukanovic R., Mueller R., Heusser C. H., Howarth P. H., Holgate S. T. Interleukin-4, -5, and -6 and tumor necrosis factor-alpha in normal and asthmatic airways: evidence for the human mast cell as a source of these cytokines. Am J Respir Cell Mol Biol. 1994 May;10(5):471–480. doi: 10.1165/ajrcmb.10.5.8179909. [DOI] [PubMed] [Google Scholar]
- Caspi R. R., Roberge F. G., McAllister C. G., el-Saied M., Kuwabara T., Gery I., Hanna E., Nussenblatt R. B. T cell lines mediating experimental autoimmune uveoretinitis (EAU) in the rat. J Immunol. 1986 Feb 1;136(3):928–933. [PubMed] [Google Scholar]
- Cockcroft D. W., Ruffin R. E., Dolovich J., Hargreave F. E. Allergen-induced increase in non-allergic bronchial reactivity. Clin Allergy. 1977 Nov;7(6):503–513. doi: 10.1111/j.1365-2222.1977.tb01481.x. [DOI] [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- Damoiseaux J. G., Döpp E. A., Beelen R. H., Dijkstra C. D. Rat bone marrow and monocyte cultures: influence of culture time and lymphokines on the expression of macrophage differentiation antigens. J Leukoc Biol. 1989 Sep;46(3):246–253. doi: 10.1002/jlb.46.3.246. [DOI] [PubMed] [Google Scholar]
- De Monchy J. G., Kauffman H. F., Venge P., Koëter G. H., Jansen H. M., Sluiter H. J., De Vries K. Bronchoalveolar eosinophilia during allergen-induced late asthmatic reactions. Am Rev Respir Dis. 1985 Mar;131(3):373–376. doi: 10.1164/arrd.1985.131.3.373. [DOI] [PubMed] [Google Scholar]
- Eidelman D. H., Bellofiore S., Martin J. G. Late airway responses to antigen challenge in sensitized inbred rats. Am Rev Respir Dis. 1988 May;137(5):1033–1037. doi: 10.1164/ajrccm/137.5.1033. [DOI] [PubMed] [Google Scholar]
- Elwood W., Lötvall J. O., Barnes P. J., Chung K. F. Characterization of allergen-induced bronchial hyperresponsiveness and airway inflammation in actively sensitized brown-Norway rats. J Allergy Clin Immunol. 1991 Dec;88(6):951–960. doi: 10.1016/0091-6749(91)90253-k. [DOI] [PubMed] [Google Scholar]
- Engel P., Huguet J., Sanosa J., Sierra P., Cols N., García-Calderón P. A. T cell subsets in allergic respiratory disease using monoclonal antibodies. Ann Allergy. 1984 Oct;53(4):337–340. [PubMed] [Google Scholar]
- Frew A. J., Moqbel R., Azzawi M., Hartnell A., Barkans J., Jeffery P. K., Kay A. B., Scheper R. J., Varley J., Church M. K. T lymphocytes and eosinophils in allergen-induced late-phase asthmatic reactions in the guinea pig. Am Rev Respir Dis. 1990 Feb;141(2):407–413. doi: 10.1164/ajrccm/141.2.407. [DOI] [PubMed] [Google Scholar]
- Frick W. E., Sedgwick J. B., Busse W. W. The appearance of hypodense eosinophils in antigen-dependent late phase asthma. Am Rev Respir Dis. 1989 Jun;139(6):1401–1406. doi: 10.1164/ajrccm/139.6.1401. [DOI] [PubMed] [Google Scholar]
- Gonzalez M. C., Diaz P., Galleguillos F. R., Ancic P., Cromwell O., Kay A. B. Allergen-induced recruitment of bronchoalveolar helper (OKT4) and suppressor (OKT8) T-cells in asthma. Relative increases in OKT8 cells in single early responders compared with those in late-phase responders. Am Rev Respir Dis. 1987 Sep;136(3):600–604. doi: 10.1164/ajrccm/136.3.600. [DOI] [PubMed] [Google Scholar]
- Gundel R. H., Letts L. G., Gleich G. J. Human eosinophil major basic protein induces airway constriction and airway hyperresponsiveness in primates. J Clin Invest. 1991 Apr;87(4):1470–1473. doi: 10.1172/JCI115155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid Q., Azzawi M., Ying S., Moqbel R., Wardlaw A. J., Corrigan C. J., Bradley B., Durham S. R., Collins J. V., Jeffery P. K. Expression of mRNA for interleukin-5 in mucosal bronchial biopsies from asthma. J Clin Invest. 1991 May;87(5):1541–1546. doi: 10.1172/JCI115166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T., Yamakawa N., Miyajima H., Maeda K., Takai S., Ueda A., Taniguchi O., Hashimoto H., Hirose S., Okumura K. An improved method for the detection of IgE antibody of defined specificity by ELISA using rat monoclonal anti-IgE antibody. J Immunol Methods. 1989 Apr 21;119(1):145–150. doi: 10.1016/0022-1759(89)90391-8. [DOI] [PubMed] [Google Scholar]
- Holt P. G., McMenamin C., Schon-Hegrad M. A., Strickland D., Nelson D., Wilkes L., Bilyk N., Oliver J., Holt B. J., McMenamin P. G. Immunoregulation of asthma: control of T-lymphocyte activation in the respiratory tract. Eur Respir J Suppl. 1991 Apr;13:6s–15s. [PubMed] [Google Scholar]
- Lemanske R. F., Jr Mechanisms of airway inflammation. Chest. 1992 Jun;101(6 Suppl):372S–377S. doi: 10.1378/chest.101.6_supplement.372s. [DOI] [PubMed] [Google Scholar]
- Martin J. G., Xu L. J., Toh M. Y., Olivenstein R., Powell W. S. Leukotrienes in bile during the early and the late airway responses after allergen challenge of sensitized rats. Am Rev Respir Dis. 1993 Jan;147(1):104–110. doi: 10.1164/ajrccm/147.1.104. [DOI] [PubMed] [Google Scholar]
- Metzger W. J., Zavala D., Richerson H. B., Moseley P., Iwamota P., Monick M., Sjoerdsma K., Hunninghake G. W. Local allergen challenge and bronchoalveolar lavage of allergic asthmatic lungs. Description of the model and local airway inflammation. Am Rev Respir Dis. 1987 Feb;135(2):433–440. doi: 10.1164/arrd.1987.135.2.433. [DOI] [PubMed] [Google Scholar]
- Moqbel R., Barkans J., Bradley B. L., Durham S. R., Kay A. B. Application of monoclonal antibodies against major basic protein (BMK-13) and eosinophil cationic protein (EG1 and EG2) for quantifying eosinophils in bronchial biopsies from atopic asthma. Clin Exp Allergy. 1992 Feb;22(2):265–273. doi: 10.1111/j.1365-2222.1992.tb03082.x. [DOI] [PubMed] [Google Scholar]
- Murphy K. R., Wilson M. C., Irvin C. G., Glezen L. S., Marsh W. R., Haslett C., Henson P. M., Larsen G. L. The requirement for polymorphonuclear leukocytes in the late asthmatic response and heightened airways reactivity in an animal model. Am Rev Respir Dis. 1986 Jul;134(1):62–68. doi: 10.1164/arrd.1986.134.1.62. [DOI] [PubMed] [Google Scholar]
- Nakajima H., Iwamoto I., Tomoe S., Matsumura R., Tomioka H., Takatsu K., Yoshida S. CD4+ T-lymphocytes and interleukin-5 mediate antigen-induced eosinophil infiltration into the mouse trachea. Am Rev Respir Dis. 1992 Aug;146(2):374–377. doi: 10.1164/ajrccm/146.2.374. [DOI] [PubMed] [Google Scholar]
- O'Byrne P. M., Dolovich J., Hargreave F. E. Late asthmatic responses. Am Rev Respir Dis. 1987 Sep;136(3):740–751. doi: 10.1164/ajrccm/136.3.740. [DOI] [PubMed] [Google Scholar]
- Olivenstein R., Renzi P. M., Yang J. P., Rossi P., Laberge S., Waserman S., Martin J. G. Depletion of OX-8 lymphocytes from the blood and airways using monoclonal antibodies enhances the late airway response in rats. J Clin Invest. 1993 Sep;92(3):1477–1482. doi: 10.1172/JCI116725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renz H., Saloga J., Bradley K. L., Loader J. E., Greenstein J. L., Larsen G., Gelfand E. W. Specific V beta T cell subsets mediate the immediate hypersensitivity response to ragweed allergen. J Immunol. 1993 Aug 15;151(4):1907–1917. [PubMed] [Google Scholar]
- Renzi P. M., Sapienza S., Waserman S., Du T., Olivenstein R., Wang N. S., Martin J. G. Effect of interleukin-2 on the airway response to antigen in the rat. Am Rev Respir Dis. 1992 Jul;146(1):163–169. doi: 10.1164/ajrccm/146.1.163. [DOI] [PubMed] [Google Scholar]
- Robinson D. S., Hamid Q., Ying S., Tsicopoulos A., Barkans J., Bentley A. M., Corrigan C., Durham S. R., Kay A. B. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992 Jan 30;326(5):298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- Romball C. G., Weigle W. O. Transfer of experimental autoimmune thyroiditis with T cell clones. J Immunol. 1987 Feb 15;138(4):1092–1098. [PubMed] [Google Scholar]
- Schuyler M., Gott K., Shopp G., Crooks L. CD3+ and CD4+ cells adoptively transfer experimental hypersensitivity pneumonitis. Am Rev Respir Dis. 1992 Dec;146(6):1582–1588. doi: 10.1164/ajrccm/146.6.1582. [DOI] [PubMed] [Google Scholar]
- Shattil S. J., Cunningham M., Hoxie J. A. Detection of activated platelets in whole blood using activation-dependent monoclonal antibodies and flow cytometry. Blood. 1987 Jul;70(1):307–315. [PubMed] [Google Scholar]
- Sirois P., Brousseau Y., Salari H., Borgeat P. Correlation between the myotropic activity of leukotriene A4 on guinea-pig lung, trachea and ileum and its biotransformation in situ. Prostaglandins. 1985 Jul;30(1):21–36. doi: 10.1016/s0090-6980(85)80008-3. [DOI] [PubMed] [Google Scholar]
- Smith M. E., Ford W. L. The recirculating lymphocyte pool of the rat: a systematic description of the migratory behaviour of recirculating lymphocytes. Immunology. 1983 May;49(1):83–94. [PMC free article] [PubMed] [Google Scholar]
- Sorkness R., Blythe S., Lemanske R. F., Jr Pulmonary antigen challenge in rats passively sensitized with a monoclonal IgE antibody induces immediate but not late changes in airway mechanics. Am Rev Respir Dis. 1988 Nov;138(5):1152–1156. doi: 10.1164/ajrccm/138.5.1152. [DOI] [PubMed] [Google Scholar]
- Sorkness R., Johns K., Castleman W. L., Lemanske R. F., Jr Late pulmonary allergic responses in actively but not passively IgE-sensitized rats. J Appl Physiol (1985) 1990 Sep;69(3):1012–1021. doi: 10.1152/jappl.1990.69.3.1012. [DOI] [PubMed] [Google Scholar]
- Walker C., Bode E., Boer L., Hansel T. T., Blaser K., Virchow J. C., Jr Allergic and nonallergic asthmatics have distinct patterns of T-cell activation and cytokine production in peripheral blood and bronchoalveolar lavage. Am Rev Respir Dis. 1992 Jul;146(1):109–115. doi: 10.1164/ajrccm/146.1.109. [DOI] [PubMed] [Google Scholar]
- Watanabe N., Ovary Z. Antigen and antibody detection by in vivo methods; a reevaluation of passive cutaneous anaphylactic reactions. J Immunol Methods. 1977;14(3-4):381–390. doi: 10.1016/0022-1759(77)90149-1. [DOI] [PubMed] [Google Scholar]