Abstract
Aim
To investigate the involvement of interleukin (IL)10 and transforming growth factor (TGF) β in the development of experimentally induced allergic conjunctivitis in mice.
Methods
Balb/c mice were actively sensitised with ragweed in alum, and then challenged with ragweed in eye drops after 10 days. 24 h later, the conjunctivas, spleens and blood were collected for histological and cytokine expression analyses, proliferation and cytokine production assays and measurement of immunoglobulin (Ig) levels. Mice developing experimentally induced allergic conjunctivitis were injected intraperitoneally with 200 μg of anti‐IL10 or anti‐TGF β antibodies at 0, 2, 4, 6 and 8 days (induction phase treatment) or 500 μg of antibodies 2 h before ragweed challenge (effector phase treatment). Normal rat IgG was used for control injections.
Results
Treatment with either anti‐IL10 or anti‐TGF β antibodies during the induction phase did not affect eosinophil infiltration into the conjunctiva. By contrast, treatment with either antibody during the effector phase suppressed infiltration. During the effector phase, treatment with anti‐TGF β antibody, but not the anti‐IL10 antibody, markedly up regulated proliferation and Th2 cytokine production by splenocytes. IL1α levels in the conjunctiva were reduced after treatment with either antibody; in addition, eotaxin and tumour necrosis factor α levels were reduced after treatment with antibody to TGF β.
Conclusions
IL10 and TGF β do not have immunosuppressive roles in the development of experimentally induced allergic conjunctivitis. Rather, they augment the infiltration of eosinophils into the conjunctiva during the effector phase of experimentally induced allergic conjunctivitis.
Severe forms of allergic conjunctivitis such as vernal keratoconjunctivitis are characterised by the formation of giant papillae in the palpebral conjunctiva.1 These papillae are formed by proliferation of fibroblasts and massive infiltration of inflammatory cells, including eosinophils.2 In addition to contributing to the formation of giant papillae, infiltrating eosinophils may lead to vision loss. In patients with atopic keratoconjunctivitis, eosinophil numbers in tear fluids increase with the severity of corneal damage,3 indicating that eosinophils have an important role in the severity of allergic conjunctivitis. Therefore, it could be considered that quantification of conjunctival infiltrating eosinophils is suitable to evaluate the severity of allergic conjunctivitis. We have investigated the mechanism by which eosinophils infiltrate into the conjunctiva during development of allergic conjunctivitis, using experimental allergic conjunctivitis (experimental immune‐mediated blepharoconjunctivitis) in rats4,5,6 and mice.7,8,9,10 Accumulating evidence has confirmed that antigen‐specific T cells (Th2 cells in particular) have a crucial role in the infiltration of eosinophils into the conjunctiva during experimental immune‐mediated blepharoconjunctivitis development.11
T cell immune responses are suppressed by immunoregulatory cytokines, of which interleukin (IL)10 and transforming growth factor (TGF) β are considered to be representative cytokines, as they are produced by regulatory T cells.12,13 There have been several investigations into the involvement of IL10 and TGF β in experimentally‐induced allergic disease. For example, administration of exogenous IL10 before allergen treatment has been shown to induce antigen‐specific T cell tolerance in an experimental dermatitis model.14 In addition, endogenous IL10 was found to suppress allergen‐induced airway inflammation;15,16 similarly, CD4+ T cells engineered to produce IL10 were shown to prevent allergen‐induced airway inflammation,17 suggesting that IL10 has an immunosuppressive role during allergic inflammation. By contrast, it was reported that IL10 promotes airway hyper‐responsiveness18 and even eosinophilia19 in allergen‐induced airway inflammation. With regard to TGF β, blocking of CTLA‐4 enhances allergic inflammation but decreases TGF β levels in bronchoalveolar lavage fluid.20 Furthermore, TGF β secreted from CD4+ T cells was found to ameliorate antigen‐induced tracheal eosinophilia.21 Thus, TGF β seems to act as a suppressive cytokine during the development of allergic airway inflammation, whereas it remains unclear whether IL10 always exhibits this function. However, so far, the roles of these two cytokines in allergic conjunctivitis have not been investigated.
In this report, to investigate the roles of IL10 and TGF β in the development of allergic conjunctivitis, we treated experimentally induced allergic conjunctivitis‐developing mice with neutralising antibodies to both cytokines.
Materials and methods
Mice
Inbred Balb/c mice were purchased from Japan SLC (Hamamatsu, Shizuoka, Japan). The mice were kept in pathogen‐free conditions at the Animal Facility of Kochi Medical School, Nankoku, Japan, and age‐matched and sex‐matched mice were used at 6–12 weeks of age. All research conformed to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
Reagents
Short ragweed pollen was purchased from Polysciences, Warrington, Pennsylvania, USA. Ragweed extract was obtained from LSL , Tokyo, Japan. Aluminium hydroxide (alum) was purchased from Sigma, St Louis, Missouri, USA. Hybridomas producing neutralising antibodies to IL10 (JES5‐2A5)22 and TGF β (1D11)23 were obtained from the ATCC. These antibodies for in vivo treatments were purified from ascites using a protein G column and contained <100 pg/ml endotoxin. For the control treatment, normal rat immunoglobulin G (nrIgG) was purchased from MP Biomedicals, Aurora, Ohio, USA.
Experimentally induced allergic conjunctivitis and treatment with antibodies during the induction phase
Fifty μl of the emulsion containing ragweed (50 μg) adsorbed to alum (675 μg) was injected into both the left hind‐footpad and the tail base of each mouse. The mice were injected intraperitoneally with 200 μg of purified anti‐IL10 (n = 10) or anti‐TGF β (n = 10) antibodies, or nrIgG (n = 10) at 0, 2, 4, 6 and 8 days after immunisation. Ten days later, the eyes of the immunised mice were challenged with ragweed in phosphate‐buffered saline (PBS; 2 mg in 10 μl per eye); 24 h later, the eyes, sera and spleens were harvested for histological and cytokine expression analyses, measurement of Ig levels, and cytokine production or proliferation assays, respectively.
Experimentally induced allergic conjunctivities and treatment with antibodies during the effector phase
Ragweed adsorbed on alum was injected into the left hind‐footpad and the tail base of each mouse as described above. Ten days later, the eyes of the immunised mice were challenged with ragweed in PBS (2 mg in 10 μl per eye). Two hours before the ragweed challenge, the mice were injected intraperitoneally with 500 μg of purified anti‐IL10 (n = 13) or anti‐TGF β (n = 13) antibodies or nrIgG (n = 13). Twenty four hours later, the eyes, sera and spleens were harvested for histological analysis, measurement of Ig levels, and T cell culture for transfer, cytokine production and proliferation assays, respectively.
Experimentally induced allergic conjunctivitis by adoptive transfer of splenocytes from actively immunised mice that had been treated with antibodies during the effector phase
Splenocytes harvested from the mice described above were cultured with ragweed extract at final concentrations of 5 μg/ml in 75 cm2 flasks at 107 cells/ml in 20 ml RPMI 1640 medium supplemented with 10% fetal calf serum ( ICN Biomedical Japan, Tokyo, Japan), 5×10−5 M 2‐mercaptoethanol, 2 mM L‐glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin. After incubation for 72 h at 37°C in a humidified atmosphere containing 5% CO2, 2×107 splenocytes were injected intraperitoneally into naive Balb/c mice (n = 8 mice per group). Four days after the transfer, the eyes of the recipient mice were challenged with ragweed in PBS (2 mg in 10 μl per eye), and 24 h later, the eyes and spleens were harvested for histological analysis.
Histological analysis
Eyes (including conjunctivas) were harvested and fixed in 10% buffered formalin, cut into horizontal 2‐μm‐thick sections and stained with Giemsa. In each section, infiltrating eosinophils in the lamina propria mucosae of the tarsal and bulbar conjunctivas were counted by two observers who were not apprised of the experimental treatment given to the samples. The sections counted were those of the central portion of the eye, which included the pupil and optic nerve head. The data are presented as the mean ± SEM per slide.
Cellular proliferation assay
Red blood cell‐depleted splenocytes (3×105 cells/well) were cultured in 96‐well flat‐bottom plates in 0.2 ml of RPMI 1640 medium supplemented with 5% fetal calf serum and 2‐mercaptoethanol. The cells were stimulated with ragweed at final concentrations of 0.2, 1, 5 and 25 μg/ml. After a 72‐h incubation at 37°C in a humidified atmosphere containing 5% CO2, the cultures were pulsed for 16 h with 0.5 μCi/well [3H]thymidine (Japan Atomic Energy Research Institute, Tokai, Japan). The cultures were then harvested and the incorporated radioactivity was measured by standard techniques. The data were expressed as change in counts per minute (cpm; mean cpm of stimulated cultures minus mean cpm of unstimulated control cultures). One representative data of four individual experiments is shown as Figures.
Measurement of total Ig levels in serum
Twenty four hours after ragweed challenge of immunised mice, blood was collected and serum prepared. Serum Ig levels were assessed using ELISA; in brief, EIA plates (Costar, Corning, New York, USA) were coated with goat anti‐mouse IgG antibody (2 μg/ml; Sigma) or affinity‐purified anti‐mouse IgE antibody (2 μg/ml; eBioscience, San Diego, California, USA). After blocking with 1% bovine serum albumin in PBS, serum samples (diluted from 1/100 to 1/10000 in PBS) were added in duplicate and incubated for 2 h at room temperature or overnight at 4°C. The plates were then washed with PBS plus 0.05% Tween 20 (PBS/T; Wako, Osaka, Japan) and incubated for 2 h at room temperature with alkaline phosphatase‐conjugated goat antibodies specific for IgG1 or IgG2a (Zymed, San Francisco, California, USA). In the case of IgE, biotin‐conjugated rat anti‐mouse IgE antibody (BD Biosciences, Franklin Lakes, New Jersey, USA) was added to each well for 2 h at room temperature. After washing, avidin‐alkaline phosphatase (Sigma) was added to each well for 1 h. After washing with PBS/T, p‐nitrophenyl phosphate (p‐nitrophenyl phosphate liquid substrate system, Sigma) was added to each well and allowed to develop for 15 min, after which absorbance was measured at 405 nm. Ig concentrations were calculated using the linear ranges of the dilution and standard curves generated with purified mouse IgG1 (BioLegend, San Diego, California, USA), mouse IgG2a (eBioscience) and mouse IgE (BD Biosciences).
Measurement of cytokines in the culture supernatants
Red blood cell‐depleted splenocytes (107 cells/ml) were cultured for 48 h with ragweed (25 μg/ml) in 96‐well flat‐bottom plates in 0.2 ml RPMI 1640 medium supplemented with 10% fetal calf serum and 2‐mercaptoethanol. Levels of IL2, IL4, IL5 and IL10 were measured using the Bioplex system (Bio‐Rad, Hercules, California, USA) according to the manufacturer's instructions. Data are shown as the average ± SEM (pg/ml) of four individual experiments.
Measurement of cytokines in the conjunctiva
Multiple cytokine levels in the conjunctiva (17 cytokines: IL1α, IL1β, IL2, IL3, IL4, IL5, IL6, IL9, IL10, IL12 (p40), IL12 (p70), IL13, IL17, G‐CSF, GM‐CSF, interferon γ and tumour necrosis factor (TNF) α; and 6 chemokines: eotaxin, KC, MCP 1, MIP 1α, MIP 1β and RANTES) were measured using the Bioplex system. The excised conjunctivas from experimentally induced allergic conjunctivitis‐developing mice treated with nrIgG, anti‐IL10 antibody, or anti‐TGF β antibody (n = 5 per group) were weighed, cut into small pieces and then homogenised in 100 μl of lysis buffer. The samples were then centrifuged at 6000 rpm for 5 min, and 50 μl of each supernatant subjected to analysis. Data are expressed as the mean ± SEM of pg cytokine/mg conjunctiva.
Statistical analysis
Differences between the anti‐IL10‐treated or anti‐TGFβ‐treated and nrIgG‐treated mice and between anti‐IL10‐treated and anti‐TGF β‐treated mice in terms of their serum IgE levels, splenocyte proliferation, cytokine production and infiltrating eosinophil numbers were tested for significance by Mann–Whitney U test. p Values <0.05 were considered to be significant.
Results
Neutralisation of IL10 or TGF β during the induction phase does not affect experimentally induced allergic conjunctivitis
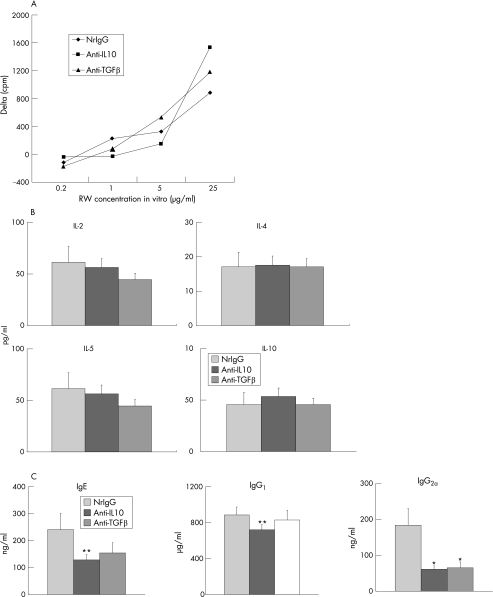
To determine whether or not IL10 and TGF β are involved in the development of experimentally induced allergic conjunctivitis during the induction phase, Balb/c mice were actively immunised with ragweed and treated with anti‐IL10 or anti‐TGF β antibodies (200 μg per time) five times (1 mg total) every other day from the day of immunisation. These mice were then challenged with ragweed‐containing eye drops on day 10. Twenty four hours after the challenge, the conjunctivas were harvested to evaluate eosinophil infiltration. Eosinophil infiltration in nrIgG‐treated group was not different from that in mice treated without any antibodies (data not shown). Compared with the control group that had been treated with nrIgG (average number of conjunctival eosinophils ± SEM = 42.0 ± 6.7), infiltration of eosinophils into the conjunctiva was not significantly different for either the anti‐IL10 antibody‐treated (42.4 ± 8.2) or anti‐TGF β antibody‐treated groups (39.9 ± 7.2). There were no significant differences in proliferation (fig 1A) or cytokine production (fig 1B) of ragweed‐stimulated splenocytes between anti‐IL10 or anti‐TGF β antibody treatments and controls (nrIgG). However, anti‐IL10 antibody treatment significantly decreased total IgE, IgG1 and IgG2a levels, whereas treatment with anti‐TGF β antibody significantly decreased total IgG2a levels (fig 1C). Thus, neutralisation of IL10 and TGF β affected systemic humoral immune responses but had no effect on eosinophil infiltration.
Figure 1 Effects of antibody treatment during the induction phase on systemic immune responses. Spleens and blood were collected to evaluate ragweed (RW)‐induced proliferation (A) and cytokine production (B) of splenocytes and total Ig levels in serum (C), respectively. (A) Data are expressed as Δ cpm. Background cpm ± SEM were: 1163 ± 22 in normal rat immunoglobulin G (nrIgG)‐treated (control) group; 985 ± 49 in the anti‐IL10 antibody‐treated group; and 1102 ± 22 in the anti‐TGF β antibody‐treated group. (B) Data are expressed as pg/ml. (C) Data are expressed as ng/ml or μg/ml. *p<0.01, **p<0.05, compared with nrIgG‐treated group.
Neutralisation of IL10 or TGF β during the effector phase suppresses experimentally induced allergic conjunctivitis
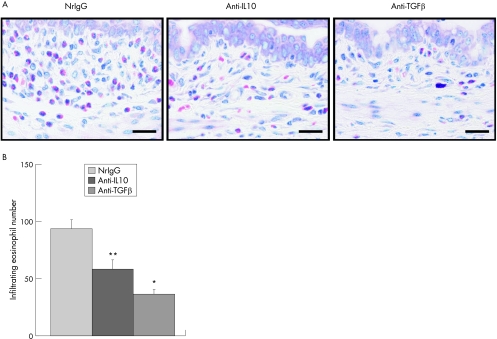
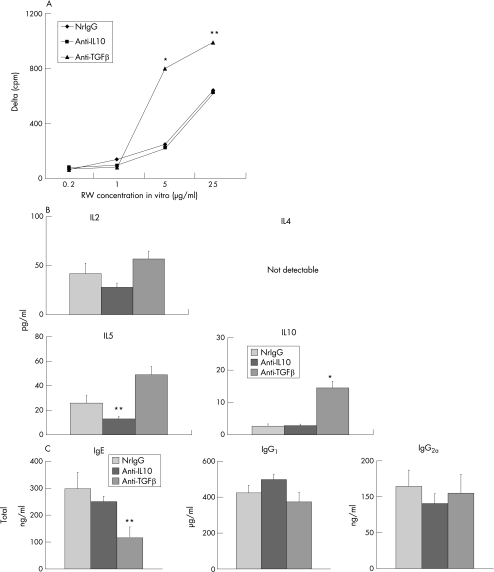
To investigate whether or not IL10 and TGF β have a role in the development of experimentally induced allergic conjunctivitis during the effector phase, immunised mice were treated with anti‐IL10 or anti‐TGF β antibodies (500 μg) 2 h before the ragweed challenge. Treatment with either antibody significantly suppressed eosinophil infiltration into the conjunctiva (fig 2A,B). Compared with the controls (nrIgG) and anti‐IL10 antibody‐treated group, treatment with anti‐TGF β antibody, significantly up‐regulated proliferation of splenocytes in response to ragweed stimulation (fig 3A). Similarly, IL2, IL5 and IL10 production by ragweed‐stimulated splenocytes was up‐regulated by treatment with anti‐TGF β antibody (fig 3B). Treatment with anti‐TGF β antibody, but not anti‐IL10 antibody, significantly decreased total IgE levels in serum compared with the controls (fig 3C). Total IgG1 and IgG2a levels in serum were unaffected by treatment with either anti‐IL10 or anti‐TGF β antibodies (fig 3C).
Figure 2 Effects of anti‐interleukin (IL)10 or anti‐transforming growth factor (TGF) β antibody treatment during the effector phase on eosinophil infiltration into the conjunctiva. Balb/c mice were actively immunised with ragweed and were then challenged with ragweed‐containing eye drops on day 10. Anti‐IL10 or anti‐TGF β antibodies (500 μg) were injected into the mice 2 h before ragweed challenge. Twenty four hours after the challenge, the conjunctivas were harvested to evaluate eosinophil infiltration. (A) Microphotograph. Bar = 20 μm. (B) Infiltrating eosinophil number. Data are presented as the mean conjunctival eosinophil number ± SEM per slide. n = 13 mice per group. *p<0.01, **p<0.05 compared with normal rat immunoglobulin (nrIgG)‐treated group.
Figure 3 Effects of antibody treatment during the effector phase on systemic immune responses. Spleens and blood were collected to evaluate ragweed (RW)‐induced proliferation (A) and cytokine production (B) of splenocytes and total Ig levels in serum (C), respectively. (A) Data are expressed as Δ cpm. Background cpm ± SEM were: 826 ± 36 in normal rat immunoglobulin G (nrIgG)‐treated (control) group; 693 ± 12 in the anti‐IL10 antibody‐treated group; and 660 ± 43 in the anti‐TGF β antibody‐treated group. (B) Data are expressed as pg/ml. (C) Data are expressed as ng/ml or μg/ml. *p<0.01, **p<0.05; , compared with the other two groups.
Splenocytes from mice treated with antibodies against IL10 or TGF β during the effector phase have equivalent potential to induce experimentally induced allergic conjunctivitis
To investigate whether or not the suppression of experimentally induced allergic conjunctivitis by treatment with anti‐IL10 antibody or anti‐TGF β antibodies is mediated by modulation of antigen‐specific cellular immune responses, we induced experimentally induced allergic conjunctivitis by adoptive transfer of splenocytes from mice treated with nrIgG (average number of conjunctival eosinophils ± SEM = 75.3 ± 13.6) or antibodies against IL10 (102.9 ± 13.4) or TGF β (106.5 ± 12.0). There were no significant differences in eosinophil infiltration into the conjunctiva between the three groups.
Treatment with antibodies against IL10 or TGF β during the effector phase decreases IL1α, eotaxin and TNF α expression in the conjunctiva
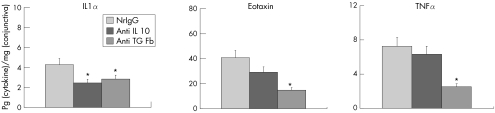
Finally, we examined the effects of treatment with antibodies against IL10 or TGF β during the effector phase on cytokine and chemokine expression in the conjunctiva. We harvested conjunctivas from experimentally induced allergic conjunctivitis‐developing mice treated with the antibodies during the effector phase and measured the levels of various cytokines and chemokines. Of the 17 cytokines and 6 chemokines examined, the levels of three molecules were affected considerably by treatment with anti‐IL10 or anti‐TGF β antibodies; compared with the control treatment (nrIgG), IL1α levels were decreased after treatment with either antibody, whereas eotaxin and TNF α levels were suppressed after treatment with anti‐TGF β antibody (fig 4).
Figure 4 Expression of cytokine and chemokine levels in the conjunctivas of experimentally induced allergic conjunctivitis‐developing mice treated with antibodies during the effector phase. Cytokine and chemokine expression levels were measured in conjunctivas harvested as described in fig 3. Of the 17 cytokines and 6 chemokines examined, IL1α, eotaxin and TNF α levels were affected by treatment with anti‐IL10 or anti‐TGF β antibodies. Data are presented as the mean ± SEM of pg cytokine/mg conjunctiva. n = 5 mice per group. *p<0.01, **p<0.05; , compared with normal rat immunoglobulin G (nrIgG)‐treated group.
Discussion
Our results show that although IL10 and TGF β do not have immunosuppressive roles in the development of experimentally induced allergic conjunctivitis, they do augment the infiltration of eosinophils into the conjunctiva during the effector phase, although these two cytokines are likely to have some different roles in systemic immune responses.
Previously, we reported that antigen‐specific cellular immune responses have a greater role in the development of experimentally induced allergic conjunctivitis (eosinophil infiltration into the conjunctiva) than do antigen‐specific humoral immune responses.7 Treatment with antibodies against IL10 or TGF β significantly affected humoral, but not antigen‐specific cellular immune responses, which may explain why treatment with these antibodies during the induction phase did not affect experimentally induced allergic conjunctivitis.
The in vitro data show that anti‐TGF β antibody treatment during the effector phase increased ragweed‐induced proliferation and production of IL5 and IL10 by splenocytes, suggesting that TGF β has an immunosuppressive role in systemic T cell responses. However, treatment with either anti‐TGF β or anti‐IL10 antibodies suppressed experimentally induced allergic conjunctivitis significantly. In addition, the splenocyte transfer results suggest that the experimentally induced allergic conjunctivitis‐inducing capability of splenocytes was not affected significantly by in vivo treatment with either antibody during the effector phase. The reason for this discrepancy remains unclear, but it may be possible that the effects of anti‐TGF β antibody are worn off in vivo during the 4‐day period after the transfer. Nevertheless, taken together, these findings suggest that attenuation of experimentally induced allergic conjunctivitis by blocking IL10 or TGF β during the effector phase is unlikely to depend on modulation of systemic cellular immune responses.
Expression of IL1α in the conjunctiva was attenuated by blocking either IL10 or TGF β. By contrast, eotaxin and TNF α expression was suppressed by the TGF β. IL1α is believed to be produced locally in the conjunctiva of patients with vernal keratoconjunctivitis.24 In patients with atopic keratoconjunctivitis, eotaxin levels in tear samples are positively correlated with the number of eosinophils in tear fluids as well as the severity of corneal damage.3 In addition, TNF α is required for upregulation of vascular permeability.25 Although it is unclear how the blocking of responses to IL10 and TGF β affects the expression of these three molecules in the conjunctiva, it may be that TGF β enhances the production of eotaxin by conjunctival fibroblasts, since it has been reported that IL13 and TGF β increase eotaxin 1 production synergistically in human airway fibroblasts.26 Taken together, our data suggest that blocking of responses to IL 10 or TGF β during the effector phase may affect the conjunctival microenvironment by decreasing cytokine or chemokine expression, thereby attenuating eosinophil infiltration into the conjunctiva. Further studies will be required to elucidate the roles of IL10 and TGF β in the regulation of eosinophil infiltration into the conjunctiva during the development of severe forms of allergic conjunctivitis.
Acknowledgements
We thank Ms. Waka Ishida and Kazuyo Fukata for their excellent technical help. This work was supported partly by grant‐in‐aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (AF).
Abbreviations
nrIgG - normal rat immunoglobulin G
PBS - phosphate‐buffered saline
TNF - tumour necrosis factor
TGF - transforming growth factor
Footnotes
Competing interests: None declared.
References
- 1.Bonini S, Coassin M, Aronni S.et al Vernal keratoconjunctivitis. Eye 200418345–351. [DOI] [PubMed] [Google Scholar]
- 2.Kumagai N, Fukuda K, Fujitsu Y.et al Role of structural cells of the cornea and conjunctiva in the pathogenesis of vernal keratoconjunctivitis. Prog Retin Eye Res 200625165–187. [DOI] [PubMed] [Google Scholar]
- 3.Fukagawa K, Nakajima T, Tsubota K.et al Presence of eotaxin in tears of patients with atopic keratoconjunctivitis with severe corneal damage. J Allergy Clin Immunol 19991031220–1221. [DOI] [PubMed] [Google Scholar]
- 4.Ozaki A, Fukushima A, Fukata K.et al Effects of IL‐4 and IL‐12 on experimental immune‐mediated blepharoconjunctivitis in Brown Norway rats. Clin Exp Immunol 200012228–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fukushima A, Fukata K, Ozaki A.et al Exertion of the suppressive effects of IFN‐gamma on experimental immune mediated blepharoconjunctivitis in brown Norway rats during the induction phase but not the effector phase. Br J Ophthalmol 2002861166–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukushima A, Ozaki A, Fukata K.et al Ag‐specific recognition, activation, and effector function of T cells in the conjunctiva with experimental immune‐mediated blepharoconjunctivitis. Invest Ophthalmol Vis Sci 2003444366–4374. [DOI] [PubMed] [Google Scholar]
- 7.Fukushima A, Ozaki A, Jian Z.et al Dissection of antigen‐specific humoral and cellular immune responses for the development of experimental immune‐mediated blepharoconjunctivitis in C57BL/6 mice. Curr Eye Res 200530241–248. [DOI] [PubMed] [Google Scholar]
- 8.Fukushima A, Yamaguchi T, Ishida W.et al Engagement of 4‐1BB inhibits the development of experimental allergic conjunctivitis in mice. J Immunol 20051754897–4903. [DOI] [PubMed] [Google Scholar]
- 9.Fukushima A, Yamaguchi T, Ishida W.et al Genetic background determines susceptibility to experimental immune‐mediated blepharoconjunctivitis: comparison of Balb/c and C57BL/6 mice. Exp Eye Res 200682210–218. [DOI] [PubMed] [Google Scholar]
- 10.Fukushima A, Yamaguchi T, Ishida W.et al Roles of OX40 in the development of murine experimental allergic conjunctivitis: exacerbation and attenuation by stimulation and blocking of OX40. Invest Ophthalmol Vis Sci 200647657–663. [DOI] [PubMed] [Google Scholar]
- 11.Ozaki A, Seki Y, Fukushima A.et al The control of allergic conjunctivitis by suppressor of cytokine signaling (SOCS)3 and SOCS5 in a murine model. J Immunol 20051755489–5497. [DOI] [PubMed] [Google Scholar]
- 12.Levings M K, Bacchetta R, Schulz U.et al The role of IL‐10 and TGF‐beta in the differentiation and effector function of T regulatory cells. Int Arch Allergy Immunol 2002129263–276. [DOI] [PubMed] [Google Scholar]
- 13.Taylor A, Verhagen J, Blaser K.et al AkdisMechanisms of immune suppression by interleukin‐10 and transforming growth factor‐β: the role of T regulatory cells. Immunology 2006117433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Enk A H, Saloga J, Becker D.et al Induction of hapten‐specific tolerance by interleukin 10 in vivo. J Exp Med 19941791397–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grunig G, Corry D B, Leach M W.et al Interleukin‐10 is a natural suppressor of cytokine production and inflammation in a murine model of allergic bronchopulmonary aspergillosis. J Exp Med 19971851089–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tournoy K G, Kips J C, Pauwels R A. Endogenous interleukin‐10 suppresses allergen‐induced airway inflammation and nonspecific airway responsiveness. Clin Exp Allergy 200030775–783. [DOI] [PubMed] [Google Scholar]
- 17.Oh J W, Seroogy C M, Meyer E H.et al CD4 T‐helper cells engineered to produce IL‐10 prevent allergen‐induced airway hyperreactivity and inflammation. J Allergy Clin Immunol 2002110460–468. [DOI] [PubMed] [Google Scholar]
- 18.Makela M J, Kanehiro A, Borish L.et al IL‐10 is necessary for the expression of airway hyperresponsiveness but not pulmonary inflammation after allergic sensitization. Proc Natl Acad Sci USA 2000976007–6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang X, Wang S, Fan Y.et al IL‐10 deficiency prevents IL‐5 overproduction and eosinophilic inflammation in a murine model of asthma‐like reaction. Eur J Immunol 200030382–391. [DOI] [PubMed] [Google Scholar]
- 20.Hellings P W, Vandenberghe P, Kasran A.et al Blockade of CTLA‐4 enhances allergic sensitization and eosinophilic airway inflammation in genetically predisposed mice. Eur J Immunol 200232585–594. [DOI] [PubMed] [Google Scholar]
- 21.Schramm C, Herz U, Podlech J.et al TGF‐beta regulates airway responses via T cells. J Immunol 20031701313–1319. [DOI] [PubMed] [Google Scholar]
- 22.Marchant A, Bruyns C, Vandenabeele P.et al Interleukin‐10 controls interferon‐gamma and tumor necrosis factor production during experimental endotoxemia. Eur J Immunol 1994241167–1171. [DOI] [PubMed] [Google Scholar]
- 23.Dasch J R, Pace D R, Waegell W.et al Monoclonal antibodies recognizing transforming growth factor‐beta. Bioactivity neutralization and transforming growth factor beta 2 affinity purification. J Immunol. 19891421536–1541. [PubMed] [Google Scholar]
- 24.Leonardi A, Borghesan F, DePaoli M.et al Procollagens and inflammatory cytokine concentrations in tarsal and limbal vernal keratoconjunctivitis. Exp Eye Res 199867105–112. [DOI] [PubMed] [Google Scholar]
- 25.Munro J M, Pober J S, Cotran R S. Tumor necrosis factor and interferon‐gamma induce distinct patterns of endothelial activation and associated leukocyte accumulation in skin of Papio anubis. Am J Pathol 1989135121–133. [PMC free article] [PubMed] [Google Scholar]
- 26.Wenzel S E, Trudeau J B, Barnes S.et al TGF‐beta and IL‐13 synergistically increase eotaxin‐1 production in human airway fibroblasts. J Immunol 20021694613–4619. [DOI] [PubMed] [Google Scholar]