Abstract
Aim
To compare the clinical findings in children with symptomatic toxoplasmic ocular lesions attributable to infection acquired before or after birth.
Methods
Cases were prospectively ascertained for 24 months through national surveillance units and reference laboratories in the British Isles. Age and presenting symptoms, site of lesion and visual impairment in children who were classified as acquiring infection either before or after birth on the basis of clinical and serological findings were compared.
Results
31 children had toxoplasmic retinochoroiditis, 15 had congenital infection and all but three of these presented before the age of 4 years. The remaining 16 children acquired toxoplasmosis postnatally, and 14 of 16 presented after the age of 10 years. A further four children had retinochoroiditis due to other causes. The presence of bilateral, multiple or posterior pole lesions did not distinguish between the two groups, but most children (16/19; 84%) presenting with acute ocular symptoms had postnatally acquired infection. Unilateral visual impairment (Snellen ⩽6/18) was equally prevalent in the two groups (4/9 before birth v 7/16 after birth; p>0.5). Only two children had bilateral visual impairment, both of whom had congenital infection. No child was blind.
Conclusions
About 50% of children with ocular lesions due to toxoplasmosis had postnatal infection. Retinochoroidal lesions due to infection before and after birth were indistinguishable. The prognosis for bilateral visual function was good, regardless of when infection was acquired.
Ophthalmologists are rarely able to distinguish between toxoplasmic retinochoroiditis due to infection acquired before or after birth, unless other clinical or serological indications are present. Epidemiological evidence suggests that most adult disease arises from infection acquired after birth.1,2 Much less is known about the prevalence of infection before and after birth in children with toxoplasmic retinochoroiditis. Two previous studies suggested that most children with ocular toxoplasmosis are infected after birth. However, one study included only five children of 83 patients with symptomatic retinochoroiditis (two of whom had congenital toxoplasmosis).1 The other study, conducted 40 years ago in a tertiary referral centre, was a case series of 28 children with acute posterior uveitis, five of whom had probable congenital toxoplasmosis.3
Knowledge of the relative contribution and severity of infection acquired before and after birth to symptomatic ocular toxoplasmosis in children would inform counselling and the public debate on the relevance of screening programmes for children.4 We therefore carried out a prospective active case surveillance study of ocular toxoplasmosis through the paediatric and ophthalmological communities in the UK, and classified children according to their likelihood of infection before and after birth. We also compared the age distribution, site of lesions and visual impairment in children.
Methods
Children with symptoms suspected to be due to toxoplasmosis were ascertained prospectively using active monthly reporting to the British Ophthalmological and British Paediatric Surveillance Units or by Referral Laboratories directly to the study coordinators. Ophthalmologists were asked to report any child presenting with unexplained retinochoroiditis or with other ocular findings (eg, microphthalmia or squint) consistent with congenital toxoplasmosis. Paediatricians and laboratories were asked to report children in whom congenital toxoplasmosis was suspected on the basis of clinical or serological findings. Clinicians who reported patients were subsequently sent a postal questionnaire to record demographic details and clinical findings based on their patient records. They were asked to record whether the child presented with acute ocular symptoms (pain, blurred vision, visual loss or floaters) or whether they were seen because of abnormal vision screening. If clinicians indicated that further information would be available (eg, from test results) from themselves or another clinician, follow‐up information was sought. All ophthalmologists were asked to give an opinion as to whether ocular manifestations were definitely, probably, possibly or not due to toxoplasmosis.
The study included children who first presented between July 2002 and June 2004 (24 months). Immunocompromised children were excluded. Two investigators (REG and MRS) independently classified all cases according to whether the ocular manifestations were probably due to toxoplasmosis and secondly, whether the infection was likely to have occurred before birth. These classifications (definite, probable, possible or not) were based on a modification of published criteria5 and incorporated the clinician's opinion where classification was equivocal (criteria given in appendix). Children with ocular (eg, microphthalmia or squint) or neurological (eg, hydrocephalus) manifestations from early childhood were assumed to have congenital infection.
We compared age and symptoms at presentation, site (posterior pole or periphery), and multiplicity of lesions and visual acuity according to whether toxoplasmosis was classified as being acquired before or after birth. Vision impairment was defined as a Snellen equivalent <6/12, as used by others.6,7
Results
Population
During the study period, 31 children were reported with definite or probable ocular toxoplasmosis, and 15 of these had definite or probable congenital toxoplasmosis. Table 1 summarises the key characteristics on which this classification was based. A further four children had retinochoroiditis due to other causes: multifocal choroiditis (n = 1), toxocara (n = 1), intermediate uveitis (n = 1) and subretinal neovascular membrane (n = 1).
Table 1 Characteristics of children classified as infected before or after birth.
| Characteristic | Congenital | Acquired | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case Number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 |
| Treatment in pregnancy | N | N | N | U | U | N | N | N | N | N | Y | N | N | N | N | U | U | U | N | N | N | U | N | U | N | N | N | U | U | N | U |
| IgM + at presentation | X | X | N | N | N | N | N | N | X | N | N | N | N | N | N | N | N | X | X | N | X | ||||||||||
| RC <2 years old | X | X | X | X | X | X | X | X | X | X | |||||||||||||||||||||
| Signs in infancy | X | X | X | X | X | X | X | X | X | X | * | ||||||||||||||||||||
| First RC ⩾⩾4–9 years | X | X | X | ||||||||||||||||||||||||||||
| First RC ⩾⩾10 years | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||||||||
IgM, immunoglobulin M; N, no; RC, retinochoroiditis; U, unknown; Y, yes.
*Amblyopia noted in the past
Age of presentation
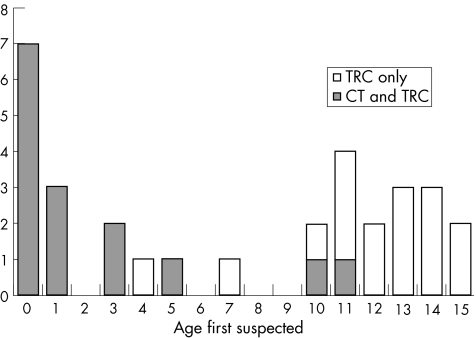
Children presented at the extremes of childhood according to whether infection was acquired before or after birth. Figure 1 shows the age of presentation of children in the study. In all, 12 of 15 children with congenital infection presented before the age of 4 years and 14 of 16 with infection acquired after birth presented at age ⩾10 years. Only three children presented between 4 and 10 years of age.
Figure 1 Age at presentation of children in the study. CT, control; TRC, toxoplasmic retinochoroiditis.
Symptoms and signs
Table 2 summarises the clinical characteristics of the children according to whether infection occurred before or after birth. Children with congenital infection were more likely to present after an abnormal vision screening test or with abnormal appearances of the eye (microphthalmia or squint). By contrast, those acquiring infection after birth all presented with acute ocular symptoms (n = 11) or acute visual impairment (n = 4) or both (n = 1).
Table 2 Presenting symptoms, history and visual impairment associated with posterior pole retinochoroiditis.
| Congenital (n = 15)* | Acquired (n = 16) | |
|---|---|---|
| Ocular symptoms at presentation | n = 14† | n = 16 |
| Abnormality at vision screening | 4 | 0 |
| Acute ocular symptoms | 3 | 16 |
| Microphthalmia or squint | 9 | 0 |
| Visual impairment in children with posterior pole lesions‡ | n = 10§ | n = 16 |
| Posterior pole lesion —bilateral | 2 | 0 |
| Visual impairment —bilateral | 2/2 | 0/0 |
| Posterior pole lesion —unilateral | 7 | 11 |
| Visual impairment in affected eye | 6/7 | 4/11 |
*One child was classified with congenital infection due to severe mental impairment although retinochoroiditis developed for the first time at 11 years of age. Consequently, toxoplasmosis could have been acquired after birth.
†Findings were not recorded for one child.
‡Impairment defined as equivalent to Snellen ⩽6/18.
§Vision not recorded for five children.
Posterior pole lesions were common, regardless of whether infection occurred before or after birth. Right and left eyes were similarly affected in both groups. Bilateral lesions occurred in four children, one of whom had postnatal infection. Unilateral visual impairment was similarly prevalent in both groups; two children had bilateral impaired vision, both in the congenitally infected group.
Discussion
About 50% of the children with ocular toxoplasmosis were considered to have acquired infection after birth. Ocular toxoplasmosis presented at the extremes of childhood, with few children reported between the age of 4 and 9 years. Children infected before birth were most likely to be detected through abnormal vision screening or ocular appearance. Children infected after birth all presented with acute ocular symptoms. The site of lesion was similar, regardless of when infection occurred. Overall, only two children had bilateral visual impairment, but no child was blind.
Our classification of the timing of infection before or after birth was based on the balance of probability using standard reproducible criteria (reported in full elsewhere)5 and was performed independently by two observers. A weakness is the limited evidence on which these criteria are based. For those classified as infected after birth, classification was necessarily based on some of the clinical manifestations (eg, presence of squint or microphthalmia). In no case did the visual acuity, site or size of lesion form any part of the assessment of timing of infection.
Information on which to base the classification of infection before or after birth was lacking in some cases. In particular, patients presenting in adolescence were unlikely to be questioned in detail about events before birth or in infancy. This is shown by the number of children in whom information on maternal infection or treatment during pregnancy was not available (table 1). The lack of data meant that, in a minority of children, our decisions were a “close call”. For instance, child number 10 presented with acute ocular symptoms at 11 years of age. She had mental retardation of unknown cause, and although no intracranial signs of congenital disease were reported, she was classified as infected before birth. Conversely, child number 16 presented with acute ocular symptoms at 14 years of age and was classified as infected after birth. He had a history of amblyopia, but there was no information on which eye was affected. In summary, our classifications were based on the balance of probability judged by two observers and may be subject to error. However, application of stricter, more specific criteria would result in a large number of children being classified as unknown and would not use all of the information available.
Our finding that most incidents of ocular toxoplasmosis in children are due to infection after birth is supported by other studies. In a population‐based study of patients presenting to ophthalmologists, we identified six children (<16 years) of whom only two were considered to have congenital infection, and timing of infection was uncertain in the remainder.1 In a case series of 29 children with ocular toxoplasmosis 40 years ago, Perkins3 classified five as due to congenital infection. The expected prevalence of ocular disease in childhood due to congenital toxoplasmosis can be estimated from the incidence of congenital infection, believed to be approximately 1 in 10 000, of whom approximately 30% will have lesions (not all symptomatic) by 12 years of age8 (overall risk 3/100 000). This overall risk is similar to the estimated burden of symptomatic ocular disease due to infection acquired after birth on the basis of the prevalence of infection during childhood (approximately 5%9) and the risk of ocular disease after acquired infection (approximately 0.5% in the first year after infection; overall risk 2.5/100 000).10,11
The age distribution showed that the presentation of ocular toxoplasmosis was most common in early childhood and after 10 years of age. Relatively few cases occurred between 4 and 9 years of age, even though the incidence of infection in these age groups would be expected to be higher than in adulthood.9 Similarly, of the 29 children reported by Perkins, two presented before 4 years, five at 8–9 years and 22 at ⩾10 years of age. These findings suggest that symptomatic ocular toxoplasmosis arising from infection acquired early in childhood may be deferred until adolescence. Further evidence to support this hypothesis comes from population‐based studies using ophthalmoscopy screening of children >5 years of age in areas of Brazil endemic with infection.12,13 Few children <10 years had ocular lesions, but by 20 years, 12%13 to 25%12 had lesions. This pattern is surprising, as most people were infected in early childhood: the seroprevalence was 50%13 to 80%12 by 10 years of age and remained at this level throughout adulthood. We hypothesise that immunological mechanisms, possibly mediated by the hormonal changes of puberty, defer the occurrence of ocular lesions until adolescence or adulthood even when infection occurs in early childhood. This hypothesis is consistent with the observation that the age distribution of symptomatic ocular toxoplasmosis is similar (typically presentation is in early adulthood (15–45 years), with a mean between 25 and 29 years) across continents despite major differences in the incidence and proportion of infections acquired during childhood.1,14,15,16
We found no other studies that have compared ocular manifestations of infection before or after birth. Others have similarly reported a good visual outcome in patients with congenitally acquired disease even in the presence of posterior pole lesions7,17
The implications of our findings for clinicians are that ocular toxoplasmosis presenting in children should not be attributed to infection before birth without supportive clinical or serological evidence. In school age children, ocular toxoplasmosis is most probably caused by infection after birth. Further work is required to design preventive strategies to avoid acquisition of infection in the childhood years and to explore the possibility that a changing hormonal milieu in adolescent years may allow the expression of symptomatic ocular disease.
Acknowledgements
We thank the clinicians who reported cases to this study, particularly Clive Edelsten, Alistair Fielder, Edward Guy, Rick Holliman, Ed Smythe and David Taylor, and Janet Francis who collated returns for the Toxoplasma Reference Unit in Swansea. Susan Cliffe contributed to the development of the study protocol. The committees of the British Paediatric Surveillance Unit and British Opthalmological Surveillance Unit made helpful comments on the design of the study.
Appendix
CASE DEFINITION
Cases were classified as probable (>50% probability) or definite congenital toxoplasma infection or toxoplasma retinochoroiditis based on the criteria of Lebech et al,5 modified as follows:
The probability of congenital toxoplasma infection was estimated from the distribution of mother to child transmission using the first positive toxoplasma antibody test date as the latest point at which seroconversion could have occurred.18 We assumed that the probability of transmission remained the same if spontaneous fetal loss occurred without clinical signs of toxoplasma infection or if signs were not known.19
Children with toxoplasmic retinochoroiditis and signs in infancy (eg, history of squint in affected eye, hydrocephalus or neurodevelopmental delay from infancy), but no serological evidence of congenital infection, were classified as probable congenital toxoplasma infection.
Children presenting from the age of 4 years onwards with toxoplasmic retinochoroiditis and no other findings were more likely to have postnatally acquired infection than congenital infection. This is based on the following assumptions: postnatal infection does not occur before 2 years, but thereafter, the rate of postnatal infection is 3.9/1000 susceptible children/year.2,15 About 1% of infected children develop symptomatic eye disease ⩾1 year after infection.2,10,15 We assumed that the incidence of congenital toxoplasma infection was 1/10 000 live births and that 10% of congenitally infected children have retinochoroiditis by 1 year, 23% by 5 years, and that all retinochoroidal lesions due to congenital infection were symptomatic.20,21
Footnotes
Funding: This work was supported by grants from the British Council for Prevention of Blindness and the British Eye Research Foundation.
Competing interests: None declared.
References
- 1.Gilbert R E, Dunn D T, Lightman S.et al Incidence of symptomatic toxoplasma eye disease: aetiology and public health implications. Epidemiol Infect 1999123283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilbert R E, Stanford M R. Is ocular toxoplasmosis due to prenatal or postnatal infection? Br J Ophthalmol 200084224–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perkins E S. Patterns of uveitis in children. Br J Ophthalmol 196650169–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hogan M J, Kimura S J, O'Connor G R. Ocular toxoplasmosis. Arch Ophthalmol 196472592–598. [DOI] [PubMed] [Google Scholar]
- 5.Lebech M, Joynson D H, Seitz H M.et al Classification system and case definitions of Toxoplasma gondii infection in immunocompetent pregnant women and their congenitally infected offspring. European Research Network on Congenital Toxoplasmosis. Eur J Clin Microbiol Infect Dis 199615799–805. [DOI] [PubMed] [Google Scholar]
- 6.Mets M B, Holfels E, Boyer K M.et al Eye manifestations of congenital toxoplasmosis. Am J Ophthalmol 1996122309–324. [DOI] [PubMed] [Google Scholar]
- 7.Brezin A P, Thulliez P, Couvreur J.et al Ophthalmic outcomes after prenatal and postnatal treatment of congenital toxoplasmosis. Am J Ophthalmol 2003135779–784. [DOI] [PubMed] [Google Scholar]
- 8.Kodjikian L, Wallon M, Fleury J.et al Ocular manifestations in congenital toxoplasmosis. Graefe's Arch Clin Exp Ophthalmol. 2005. update from web [DOI] [PubMed]
- 9.Ades A E, Nokes D J. Modelling age and time specific incidence from seroprevalence: toxoplasmosis. Am J Epidemiol 19931371022–1034. [DOI] [PubMed] [Google Scholar]
- 10.Burnett A J, Shortt S G, Isaac Renton J.et al Multiple cases of acquired toxoplasmosis retinitis presenting in an outbreak. Ophthalmology 19981081032–1037. [DOI] [PubMed] [Google Scholar]
- 11.Perkins E S. Ocular toxoplasmosis. Br J Ophthalmol 1973571–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glasner P D, Silveira C, Kruszon‐Moran D.et al An unusually high prevalence of ocular toxoplasmosis in Southern Brazil. Am J Ophthalmol 1992114136–144. [DOI] [PubMed] [Google Scholar]
- 13.Portela R W D, Bethony J, Costa M I.et al A multihousehold study reveals a positive correlation between age, severity of ocular toxoplasmosis, and levels of glycoinositophospholipid‐specific immunoglobulin A. J Infect Dis 2004190175–183. [DOI] [PubMed] [Google Scholar]
- 14.Melamed J. The ocular features of toxoplasmosis. In: Dernouchamps JP, Verougstraete C, Caspers‐Velu L, Tassignon MJ, eds. Recent advances in uveitis. Amsterdam: Kugler Publications, 1993283–288.
- 15.Welton N J, Ades A E. A model of toxoplasmosis incidence in the UK: evidence synthesis and consistency of evidence. J R Stat Soc (C) Appl Statist 200554385–404. [Google Scholar]
- 16.Bosch‐Driessen L E, Berendschot T T, Ongkosuwito J V.et al Ocular toxoplasmosis: clinical features and prognosis of 154 patients. Ophthalmology 2002109869–878. [DOI] [PubMed] [Google Scholar]
- 17.Lappalainen M, Koskiniemi M, Hiilesmaa V.et al Outcome of children after maternal primary Toxoplasma infection during pregnancy with emphasis on avidity of specific IgG. The Study Group. Pediatr Infect Dis J 199514354–361. [DOI] [PubMed] [Google Scholar]
- 18.Gilbert R, Gras L. Effect of timing and type of treatment on the risk of mother to child transmission of Toxoplasma gondii. BJOG 2003110112–120. [DOI] [PubMed] [Google Scholar]
- 19.Freeman K, Oakley L, Pollak A.et al Congenital toxoplasmosis and preterm birth, low birth weight, and small for gestational age birth. BJOG 200411231–37. [DOI] [PubMed] [Google Scholar]
- 20.Gras L, Gilbert R E, Ades A E.et al Effect of prenatal treatment on the risk of intracranial and ocular lesions in children with congenital toxoplasmosis. Int J Epidemiol 2001301309–1313. [DOI] [PubMed] [Google Scholar]
- 21.Binquet C, Wallon M, Quantin C.et al Prognostic factors for the long‐term development of ocular lesions in 327 children with congenital toxoplasmosis. Epidemiol Infect 20031311157–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koppe J G, Loewer Sieger D H, de Roever Bonnet H. Results of 20‐year follow‐up of congenital toxoplasmosis. Lancet 1986i254–256. [DOI] [PubMed] [Google Scholar]
- 23.Peyron F, Wallon M, Bernardoux C. Long‐term follow‐up of patients with congenital ocular toxoplasmosis. N Engl J Med 1996334993–994. [DOI] [PubMed] [Google Scholar]
- 24.Gilbert R E, Peckham C S. Congenital toxoplasmosis in the United Kingdom: to screen or not to screen. J Med Screen 20029135–141. [DOI] [PubMed] [Google Scholar]