Abstract
Decades of research on vascular endothelial growth factor (VEGF) have reached fruition with the recent development of intravitreal anti‐VEGF treatments for exudative age‐related macular degeneration. VEGF is a critical regulator of angiogenesis and vascular permeability with diverse roles, both pathological and physiological, during development and adulthood. The aim of this article is to review aspects of VEGF biology that may be relevant to the clinical use of anti‐VEGF agents in ophthalmology: molecular characteristics and isoforms of VEGF; its roles in vasculogenesis, vascular maintenance and angiogenesis; systemic effects of VEGF inhibition; and properties of current anti‐VEGF agents.
The advent of anti‐vascular endothelial growth factor (VEGF) treatments marks a major advancement in the treatment of angiogenic eye disease, first with the US Food and Drug Administration approval of pegaptanib sodium (Macugen; OSI/Eyetech Pharmaceuticals, New York, USA) in 2004, followed by recent positive clinical trial results for ranibizumab (Lucentis; Genentech) and the growing off‐label use of intravitreal (ITV) bevacizumab (Avastin; Genentech, San Francisco, USA). Although it is clear from randomised clinical trials that ITV anti‐VEGF treatments will have a fundamental effect for patients with neovascular age‐related macular degeneration (AMD), ophthalmologists embarking on the use of these drugs are still faced with some unresolved issues. These include determining the ideal regimen and duration of treatment, the potential of combination treatments and safety concerns with long‐term VEGF inhibition. Although some of these issues may be tackled by expanding clinical experience, a review of the relevant VEGF science can inform the clinical application of these novel treatments. The aim of this review is to consider the biology of the VEGF gene family and VEGF‐A isoforms, the physiological role of VEGF‐A in development and adulthood, the role of VEGF‐A in the pathogenesis of ocular diseases, the differential therapeutic mechanisms of current and upcoming anti‐VEGF agents and the safety considerations involved in VEGF inhibition.
VEGF biology
VEGF is a homodimeric glycoprotein and is a growth factor specific for endothelial cells.1 It is a critical regulator of vasculogenesis and angiogenesis, as well as a potent inducer of vascular permeability.2,3,4 Additional VEGF functions under study include retinal leukostasis and neuroprotection.2,3,5,6,7 Three receptor tyrosine kinases have been identified for VEGF: VEGF receptor (VEGFR)1 (fms‐like tyrosine kinase‐1) has both positive and negative angiogenic effects; VEGFR2 (fetal liver kinase‐1 and kinase insert domain‐containing receptor) is the primary mediator of the mitogenic, angiogenic and vascular permeability effects of VEGF‐A; and VEGFR3 mediates the angiogenic effects on lymphatic vessels.1,8 The pathological functions of VEGF‐A have received the most attention, culminating in the development of a new class of drugs for neovascular eye disease and cancers. But VEGF‐A and its receptors are also present in tissues and organ systems in normal adults, underlining the physiological role of VEGF‐A.
VEGF gene family
The VEGF gene family consists of VEGF‐A, VEGF‐B, VEGF‐C, VEGF‐D and placental growth factor (PlGF), which have different binding affinities for the three VEGF receptors.9,10 VEGF‐A is the best studied, has been most strongly associated with angiogenesis, and is the target of most current anti‐VEGF treatments.11,12 VEGF‐A signals through two receptor tyrosine kinases, VEGFR1 and VEGFR2, and is the only member of the VEGF gene family found to be induced by hypoxia.13 VEGF‐B selectively binds VEGFR1 and has a role in the regulation of extracellular matrix degradation, cell adhesion and migration.9 Both VEGF‐C and VEGF‐D bind VEGFR2 and VEGFR3 and regulate lymphangiogenesis, and VEGF‐C may also be involved in wound healing.9,10,14 PlGF selectively binds VEGFR19 and is the most abundantly expressed VEGF family member in endothelial cells.13 PlGF may potentiate VEGF‐A‐induced endothelial cell proliferation,13 but on its own PlGF exerts only weak mitogenicity.13
VEGF‐A isoforms
Nine major VEGF‐A isoforms have been identified in humans: VEGF121, VEGF145, VEGF148, VEGF162, VEGF165, VEGF165b (an endogenous inhibitory isoform that binds to VEGFR2 with similar affinity to VEGF165 but does not activate it), VEGF183, VEGF189, and VEGF206.15 These isoforms are produced by alternative exon splicing of the human VEGF‐A gene on chromosome 6p21.3.1,15 VEGF165 and longer isoforms consist of two domains: a VEGF receptor‐binding domain that is present in all VEGF‐A isoforms and a heparin‐binding domain that is absent in the shorter diffusible isoforms. Longer isoforms, such as VEGF189 and VEGF206, bind to the extracellular matrix (ECM) via the heparin‐binding domain.1,16 The VEGF165 isoform is intermediate, and exists as both diffused and partly ECM‐bound.17,18 Studies have established that VEGF165 is the most abundantly expressed VEGF‐A isoform and has a vital role in angiogenesis.19,20 In addition, one study has found that VEGF121, although less abundant, is more mitogenic than VEGF165 or VEGF189.21 All VEGF‐A isoforms except VEGF121 contain a plasmin cleavage site and theoretically may be cleaved by plasmin to generate the smaller VEGF110 form.15,19 VEGF110 can stimulate endothelial cell growth and induce vascular permeability in the Miles assay17; however, its mitogenic potency is less than that of VEGF165.22
VEGF121, VEGF165, VEGF183 and VEGF189 are distributed widely in tissue, with VEGF165 expression the most abundant. In contrast, VEGF145 and VEGF206 are less abundant. In mice the VEGF‐A gene is highly conserved, with three splice variants, VEGF120, VEGF164 and VEGF188, equivalent to the human VEGF121, VEGF165 and VEGF189 isoforms, respectively.23
VEGF‐A in vasculogenesis
Vascular formation can be defined in two distinct categories: vasculogenesis and angiogenesis. Vasculogenesis is the process of de novo blood vessel formation during embryogenesis,24 which begins with the differentiation, proliferation and migration of haemangiogenic stem cells under the possible influence of VEGF‐A and PlGF.25 VEGF‐A may also participate in endothelial cell survival and in capillary regression via synchronous apoptosis.26
Angiogenesis, which can occur in adulthood, is the process of neovascularisation from established blood vessels. Angiogenesis occurs in response to a variety of pathological stimuli including ischaemia, inflammation, wound healing and tumour formation. Angiogenesis can also be integral to tissue homoeostasis and normal processes such as the growth and maintenance of the ovarian follicle and corpus luteum during reproductive life in women.27 Thus, angiogenesis in adulthood can have both detrimental effects, such as neovascular AMD, rheumatoid arthritis and cancer,12 and beneficial effects, such as cardiac remodelling and wound healing.
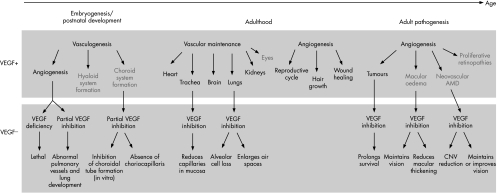
VEGF‐A is integral to blood vessel formation during both vasculogenesis and angiogenesis, but its role differs in development versus adulthood (fig 1). For instance, in developing mice, newly formed vessels require VEGF‐A to persist through the fourth postnatal week, but after that time the vessels stabilise and no longer depend on VEGF‐A for maintenance.28 VEGF deficiency is lethal during embryogenesis.11 Postnatal inhibition of the VEGF‐A gene via conditional knockout technology increases mortality.28 Data from selective VEGF‐A isoform knockout mice suggest that the absence of VEGF164 and VEGF188 results in a variety of vascular defects, including abnormal pulmonary vascular development.23
Figure 1 The role of VEGF‐A and the effect of VEGF inhibition in embryogenesis, postnatal development and adulthood under normal and pathological conditions, particularly in the ocular system. AMD, age‐related macular degeneration; CNV, choroidal neovascularisation; VEGF, vascular endothelial growth factor.
VEGF‐A in ocular development
During ocular development, vasculogenesis occurs firstly in the choroid and hyaloid systems, where VEGF‐A may have a role. VEGF‐A expression has been implicated in the abnormal persistence of the hyaloid vasculature in a mouse model of persistent fetal vasculature.29 In VEGFrpe−/− mice, the lack of VEGF‐A expression relegated specifically to the retinal pigment epithelium (RPE) results in failure of the choriocapillaris to form.30 Vascularisation of the retina occurs later in embryogenesis and is not completed until the final postnatal period,31 with VEGF‐A controlling vessel sprouting and migration in the postnatal retina.32 VEGF‐A expressed in the neural retina is correlated temporally and spatially with normal vasculogenesis in both humans24 and rats.33 VEGF‐A appears to be induced by physiological hypoxia as part of normal retinal development, and VEGF‐A suppression inhibits vascular formation.31
The function of the individual VEGF‐A isoforms may differ during ocular development. Mice expressing only VEGF164 have normal retinal angiogenesis and vasculature, whereas mice expressing only VEGF120 or VEGF188 have aberrant vascular outgrowth and arterial development.34 VEGF164 shows the greatest upregulation during rat ocular development, in the presence of VEGF120, VEGF164 and VEGF188.35
VEGF‐A in the maintenance of adult vasculature
In addition to vasculogenesis and angiogenesis, VEGF‐A may participate in the maintenance of certain vascular systems in the adult. Specific binding of VEGF‐A is associated with mature vessels in various adult rat tissues, such as the heart, kidney and brain, suggesting that VEGF‐A has a function in the maintenance of quiescent vascular endothelium.36 VEGF‐A maintains normal tracheal vasculature in adult mice, as shown by capillary loss after broad VEGF inhibition, an effect not seen with selective VEGF164 inhibition.37 In addition, chronic VEGF inhibition in adult rats results in lung alveolar septal abnormalities.38
VEGF‐A in the maintenance of adult ocular vasculature
Little is known of the role of VEGF‐A in the maintenance of adult ocular vasculature. VEGF‐A is produced by human differentiated RPE cells both in vivo and in vitro, and may be involved in the paracrine signalling between the RPE and choriocapillaris.39 In normal adult monkey and mouse eyes, the VEGF121 and VEGF165 isoforms are most abundantly expressed in the choroid, RPE, retina and iris tissue, consistent with VEGF‐A acting as a vascular survival factor in the adult eye.40 However, in adult mice treated systemically with vatalanib, a broad inhibitor of VEGF‐A isoforms and platelet‐derived growth factor, no effects are seen in the retinal vasculature.41 To date, clinical trials of the VEGF‐A inhibitors pegaptanib and ranibizumab in humans have not shown adverse effects on normal retinal or choroidal vasculature (Rosenfeld et al41a; Brown et al41b).42
VEGF‐A in ocular pathogenesis
The central role of VEGF‐A is well established in ocular neovascular diseases.43,44,45,46 In humans, high levels of VEGF‐A expression are found in choroidal neovascularisation (CNV) tissue excised from patients with AMD.43,47 Intraocular VEGF‐A levels correlate with blood vessel formation in patients with diabetic retinopathy and other retinal disorders.44,48
Different VEGF‐A isoforms may have different functions in ocular diseases. VEGF164 is the predominant isoform expressed at the time of maximal preretinal neovascularisation in a neonatal rat model35 and is the primary proinflammatory isoform in the retina of rats with diabetes.49,50 Levels of both VEGF121 and VEGF165 are increased in monkeys after laser‐induced retinal vein occlusion.51 VEGF120 is the main isoform expressed in mouse CNV membranes, and inhibition of VEGF120 results in reduction of CNV in mice.43,52 Both VEGF121 and VEGF165 isoforms are found in CNV tissue excised from patients with AMD.43
VEGF‐A inhibition
Anti‐VEGF agents
Currently available and emerging anti‐VEGF treatments inhibit VEGF‐A isoforms in different ways (summarised in fig 2). Pegaptanib sodium is a pegylated ribonucleic acid oligonucleotide ligand (or aptamer). It has a molecular mass of 50 kDa and is produced synthetically to be specific for the human VEGF164 isoform, binding at its heparin‐binding domain.16 Theoretically, it may bind the longer VEGF‐A isoforms that also contain the heparin‐binding domain.
Figure 2 Key angiogenesis activators and inhibitors. Note that only the four major vascular endothelial growth factor (VEGF)‐A isoforms are depicted in this schematic representation. ECM, extracellular matrix; TKIs, tyrosine kinase inhibitors; VEGFR, vascular endothelial growth factor receptor.
Ranibizumab is a humanised antigen‐binding fragment of a murine full‐length monoclonal antibody (mAB) directed against human VEGF‐A. It is produced in Escherichia coli using recombinant DNA technology and has a molecular mass of 48 kDa. Ranibizumab has been shown to inhibit VEGF165, VEGF121 and VEGF110 (Lowe et al, unpublished data), and theoretically should inhibit all VEGF‐A isoforms and their active degradation products, although it is technically difficult to evaluate the activity and binding of longer VEGF‐A isoforms because they are sequestered in the ECM. Ranibizumab fully penetrates the retinal layers to the outer retina and inner choroid in rabbits (Gaudreault et al, unpublished data).
Bevacizumab is a 149‐kDa full‐length humanised mAB against all VEGF‐A isoforms and their active degradation products, approved as intravenous infusion in combination with chemotherapy for metastatic colorectal cancer. Off‐label use of ITV bevacizumab for neovascular AMD in small retrospective studies has shown benefits for vision and reduced macular thickening.53,54 The long‐term benefits and safety of ITV bevacizumab remain to be investigated.
Several other anti‐VEGF treatments are in clinical development for ophthalmology. VEGF Trap (Regeneron Pharmaceuticals, New York, USA) is a fusion protein containing the binding sites of VEGFR1 and VEGFR2, which inhibits all VEGF‐A isoforms and PlGF.55 Receptor tyrosine kinase inhibitors include vatalanib (PTK787; Novartis AG) and AG‐013958 (Pfizer, New York, USA); these target the VEGF receptor tyrosine kinases and inhibit all VEGF‐A isoforms,56 although AG‐013958 also inhibits platelet‐derived growth factor receptors. RNA interference‐based treatments are also being explored in neovascular AMD. Cand5 (Acuity Pharmaceuticals, Philadelphia, USA) prevents the production of all VEGF‐A isoforms by degrading VEGF‐A mRNA. Sirna‐027 (Sirna Therapeutics, Boulder, USA) targets production of VEGFR1 mRNA.57
Therapeutic outcomes
In the phase III VGEF Inhibition Study in Ocular Neovascularization (VISION) clinical trials, pegaptanib has been shown to be beneficial for all subtypes of CNV secondary to AMD. At 12 months, considerably more pegaptanib‐treated patients lost <15 letters than patients who received sham injections (70% v 55%) and more patients gained ⩾3 lines of visual acuity (6% v 2%)42; benefits were maintained at 24 months. Rates of visual gain might be enhanced for treatment of small and early CNV lesions, as suggested by a subanalysis of a small group of patients from the VISION trial.58 For patients with diabetic macular oedema, a phase II controlled clinical trial showed a considerably higher rate of visual gain of ⩾10 letters of visual acuity for pegaptanib‐treated patients compared with sham‐injected patients (34% v 10%).59
In the phase III MARINA trial of patients with occult or minimally classic subfoveal CNV secondary to AMD, at month 12 considerably more ranibizumab‐treated patients lost <15 letters than sham‐injected patients (95% v 62%). Of the ranibizumab‐treated patients, 25% and 34% (0.3 and 0.5 mg, respectively) gained ⩾15 letters compared with 5% of sham‐injected patients, a significant difference (Rosenfeld et al41). In the phase III ANCHOR trial of patients with predominantly classic subfoveal CNV secondary to AMD, 94% and 96% of ranibizumab‐treated patients (0.3 and 0.5 mg, respectively) lost <15 letters of visual acuity compared with 64% of photodynamic therapy‐treated patients in month 12 (Brown et al41a). Of the ranibizumab‐treated patients, 36% and 40% (0.3 and 0.5 mg, respectively) gained ⩾15 letters compared with 6% of photodynamic therapy‐treated patients, a significant difference (Brown et al41b).
Serious ocular adverse events were rare for both pegaptanib42 and ranibizumab (Rosenfeld et al41a; Brown et al41b), and were predominantly related to the ITV injection procedure itself, including presumed endophthalmitis, traumatic lens injury and retinal detachment.42 Serious uveitis occurred at a rate of 0–1% in pegaptanib‐treated patients in the VISION trial, and at a rate of 0.7–0.8% in ranibizumab‐treated patients in the MARINA and ANCHOR trials (Rosenfeld et al41a; Brown et al41b). There is a theoretical possibility of untoward effects with long‐term VEGF‐A inhibition, particularly on normal choroidal and retinal vasculature. However, such toxicities have not been observed to this point in human clinical trials of ocular anti‐VEGF treatments.
Systemic risks with VEGF‐A inhibition
Intravenous infusion
For patients with cancer, adjuvant bevacizumab treatment in phase III clinical trials improves median survival in patients with metastatic colorectal cancer (CRC)60 and improves response rate (but not progression‐free survival) in patients with metastatic breast cancer.61 In these patients with late‐stage cancer receiving serial intravenous infusion of bevacizumab at doses of 5–15 mg/kg, there were increased risks of systemic adverse events. Compared with chemotherapy alone, bevacizumab in combination with chemotherapy is associated with an increased incidence of hypertension, bleeding and proteinuria in patients with CRC62 and an increased rate of thromboembolic events, gastrointestinal perforations, myocardial infarction and death in patients with breast cancer and CRC.61,62
A different serious adverse‐event profile has been seen in the limited number of patients without cancer studied with intravenous bevacizumab used as monotherapy. In a small study of off‐label intravenous infusion of bevacizumab in patients with AMD, seven of nine patients experienced hypertension controllable with drugs, but no serious ocular or systemic adverse events were reported.63 Similarly, no ocular or systemic adverse events were reported in two patients with pathological myopia treated off‐label with intravenous infusion of bevacizumab.64
Intravitreal administration
Given the risks of intravenous adjuvant bevacizumab treatment seen in patients with cancer, systemic risks also theoretically exist with ITV administration of anti‐VEGF treatments. Although the dosages in ocular therapy are several orders of magnitude lower, ITV injection does lead to detectable levels in the serum. In monkeys the vitreous half life (t1/2) of ranibizumab is 3 days, and after ITV injection of 0.5 mg, the maximum serum level is 150 ng/ml, with a serum half life of 3.5 days.65 In human studies, the mean (SD) serum concentration of ranibizumab 1 h after ITV administration (0.3 mg) was 1.01 (1.35) ng/ml, and after 28 days serum concentrations were <0.300 ng/ml in 96% of the patients.66 Such levels of serum ranibizumab are below the approximately 10 ng/ml threshold estimated to affect VEGF‐A‐related activity in humans.66,67
The serum half life of pegaptanib after ITV administration (3 mg) in humans is 10 days, with a mean maximum serum concentration of pegaptanib of 80 ng/ml.68 In contrast, the serum half life of the full‐length anti‐VEGF antibody bevacizumab is 17–21 days in humans; mABs tend to have relatively longer half lives than antigen‐binding fragments.69
The various VEGF‐A isoforms are distributed widely in tissue; theoretically, broad inhibition of VEGF‐A isoforms confers higher non‐specificity and systemic risk, as does prolonged serum half life. To date, more than 2000 patients have received serial ITV injections in the controlled clinical trials of pegaptanib and ranibizumab without showing increased risk of systemic adverse events for either agent. The systemic safety of ITV bevacizumab is not established, but no known serious adverse events have been reported in uncontrolled studies to date.53,54,70,71 As the clinical use of ITV VEGF inhibition expands, ongoing monitoring for systemic adverse events may be warranted.
Conclusions
Anti‐VEGF agents are the first biological compounds to be developed specifically for retinal therapeutics. The retinal community is currently dealing with aspects of these novel drugs in the management of patients with neovascular AMD. Unresolved clinical issues include: optimal injection frequency; duration of the treatment regimen; possible combination treatments; and potential applications for retinal diseases beyond AMD. In light of the role of VEGF‐A in diverse organ systems in normal adults, long‐term VEGF‐A suppression raises theoretical ocular and systemic safety issues; however, no evidence of such increased risks has been observed in controlled clinical trials of pegaptanib or ranibizumab for patients with neovascular AMD. VEGF‐A is perhaps the most widely researched molecule in ophthalmology; as such, VEGF science may be valuable to doctors in refining the clinical application of this new class of drugs.
Acknowledgements
This project was supported by “That Man May See Foundation”. Writing and research assistance was supported by Genentech.
Abbreviations
AMD - age‐related macular degeneration
CNV - choroidal neovascularisation
CRC - colorectal cancer
ECM extracellular matrix -
ITV - intravitreal
mAB - monoclonal antibody
PlGF - placental growth factor
RPE - retinal pigment epithelium
VEGF - vascular endothelial growth factor
VEGFR - VEGF receptor
VISION - VEGF Inhibition Study in Ocular Neovascularization
Footnotes
Competing interests: None declared.
References
- 1.Ferrara N, Gerber H P, LeCouter J. The biology of VEGF and its receptors. Nat Med 20039669–676. [DOI] [PubMed] [Google Scholar]
- 2.Senger D R, Galli S J, Dvorak A M.et al Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 1983219983–985. [DOI] [PubMed] [Google Scholar]
- 3.Leung D W, Cachianes G, Kuang W J.et al Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 19892461306–1309. [DOI] [PubMed] [Google Scholar]
- 4.Ferrara N, Henzel W J. Pituitary follicular cells secrete a novel heparin‐binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun 1989161851–858. [DOI] [PubMed] [Google Scholar]
- 5.Miyamoto K, Khosrof S, Bursell S E.et al Vascular endothelial growth factor (VEGF)‐induced retinal vascular permeability is mediated by intercellular adhesion molecule‐1 (ICAM‐1). Am J Pathol 20001561733–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azzouz M, Ralph G S, Storkebaum E.et al VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature 2004429413–417. [DOI] [PubMed] [Google Scholar]
- 7.Ding X M, Mao B Y, Jiang S.et al Neuroprotective effect of exogenous vascular endothelial growth factor on rat spinal cord neurons in vitro hypoxia. Chin Med J (Engl) 20051181644–1650. [PubMed] [Google Scholar]
- 8.Karkkainen M J, Makinen T, Alitalo K. Lymphatic endothelium: a new frontier of metastasis research. Nat Cell Biol 20024E2–E5. [DOI] [PubMed] [Google Scholar]
- 9.Olofsson B, Korpelainen E, Pepper M S.et al Vascular endothelial growth factor B (VEGF‐B) binds to VEGF receptor‐1 and regulates plasminogen activator activity in endothelial cells. Proc Natl Acad Sci USA 19989511709–11714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bauer S M, Bauer R J, Liu Z J.et al Vascular endothelial growth factor‐C promotes vasculogenesis, angiogenesis, and collagen constriction in three‐dimensional collagen gels. J Vasc Surg 200541699–707. [DOI] [PubMed] [Google Scholar]
- 11.Carmeliet P, Ferreira V, Breier G.et al Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 1996380435–439. [DOI] [PubMed] [Google Scholar]
- 12.Ferrara N. Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: therapeutic implications. Semin Oncol 20022910–14. [DOI] [PubMed] [Google Scholar]
- 13.Yonekura H, Sakurai S, Liu X.et al Placenta growth factor and vascular endothelial growth factor B and C expression in microvascular endothelial cells and pericytes. Implication in autocrine and paracrine regulation of angiogenesis. J Biol Chem 199927435172–35178. [DOI] [PubMed] [Google Scholar]
- 14.Stacker S A, Caesar C, Baldwin M E.et al VEGF‐D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med 20017186–191. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi H, Shibuya M. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin Sci (Lond) 2005109227–241. [DOI] [PubMed] [Google Scholar]
- 16.Lee J H, Canny M D, De Erkenez A.et al A therapeutic aptamer inhibits angiogenesis by specifically targeting the heparin binding domain of VEGF165. Proc Natl Acad Sci USA 200510218902–18907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Houck K A, Leung D W, Rowland A M.et al Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J Biol Chem 199226726031–26037. [PubMed] [Google Scholar]
- 18.Park J E, Keller G A, Ferrara N. The vascular endothelial growth factor (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix‐bound VEGF. Mol Biol Cell 199341317–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keyt B A, Berleau L T, Nguyen H V.et al The carboxyl‐terminal domain (111‐165) of vascular endothelial growth factor is critical for its mitogenic potency. J Biol Chem 19962717788–7795. [DOI] [PubMed] [Google Scholar]
- 20.Soker S, Takashima S, Miao H Q.et al Neuropilin‐1 is expressed by endothelial and tumor cells as an isoform‐specific receptor for vascular endothelial growth factor. Cell 199892735–745. [DOI] [PubMed] [Google Scholar]
- 21.Zhang H T, Scott P A, Morbidelli L.et al The 121 amino acid isoform of vascular endothelial growth factor is more strongly tumorigenic than other splice variants in vivo. Br J Cancer 20008363–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fairbrother W J, Champe M A, Christinger H W.et al Solution structure of the heparin‐binding domain of vascular endothelial growth factor. Structure 19986637–648. [DOI] [PubMed] [Google Scholar]
- 23.Ng Y S, Rohan R, Sunday M E.et al Differential expression of VEGF isoforms in mouse during development and in the adult. Dev Dyn 2001220112–121. [DOI] [PubMed] [Google Scholar]
- 24.Gogat K, Le Gat L, Van Den Berghe L.et al VEGF and KDR gene expression during human embryonic and fetal eye development. Invest Ophthalmol Vis Sci 2004457–14. [DOI] [PubMed] [Google Scholar]
- 25.Demir R, Kayisli U A, Cayli S.et al Sequential steps during vasculogenesis and angiogenesis in the very early human placenta. Placenta 200627535–539. [DOI] [PubMed] [Google Scholar]
- 26.Meeson A P, Argilla M, Ko K.et al VEGF deprivation‐induced apoptosis is a component of programmed capillary regression. Development 19991261407–1415. [DOI] [PubMed] [Google Scholar]
- 27.Gordon J D, Mesiano S, Zaloudek C J.et al Vascular endothelial growth factor localization in human ovary and fallopian tubes: possible role in reproductive function and ovarian cyst formation. J Clin Endocrinol Metab 199681353–359. [DOI] [PubMed] [Google Scholar]
- 28.Gerber H P, Hillan K J, Ryan A M.et al VEGF is required for growth and survival in neonatal mice. Development 19991261149–1159. [DOI] [PubMed] [Google Scholar]
- 29.Martin A C, Thornton J D, Liu J.et al Pathogenesis of persistent hyperplastic primary vitreous in mice lacking the arf tumor suppressor gene. Invest Ophthalmol Vis Sci 2004453387–3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marneros A G, Fan J, Yokoyama Y.et al Vascular endothelial growth factor expression in the retinal pigment epithelium is essential for choriocapillaris development and visual function. Am J Pathol 20051671451–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stone J, Itin A, Alon T.et al Development of retinal vasculature is mediated by hypoxia‐induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci 1995154738–4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerhardt H, Golding M, Fruttiger M.et al VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol 20031611163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murata T, Nakagawa K, Khalil A.et al The temporal and spatial vascular endothelial growth factor expression in retinal vasculogenesis of rat neonates. Lab Invest 19967468–77. [PubMed] [Google Scholar]
- 34.Stalmans I, Ng Y S, Rohan R.et al Arteriolar and venular patterning in retinas of mice selectively expressing VEGF isoforms. J Clin Invest 2002109327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McColm J R, Geisen P, Hartnett M E. VEGF isoforms and their expression after a single episode of hypoxia or repeated fluctuations between hyperoxia and hypoxia: relevance to clinical ROP. Mol Vis 200410512–520. [PMC free article] [PubMed] [Google Scholar]
- 36.Jakeman L B, Winer J, Bennett G L.et al Binding sites for vascular endothelial growth factor are localized on endothelial cells in adult rat tissues. J Clin Invest 199289244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baffert F, Le T, Sennino B.et al Cellular changes in normal blood capillaries undergoing regression after inhibition of VEGF signaling. Am J Physiol Heart Circ Physiol 2006290H547–H559. [DOI] [PubMed] [Google Scholar]
- 38.Kasahara Y, Tuder R M, Taraseviciene‐Stewart L.et al Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest 20001061311–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blaauwgeers H G, Holtkamp G M, Rutten H.et al Polarized vascular endothelial growth factor secretion by human retinal pigment epithelium and localization of vascular endothelial growth factor receptors on the inner choriocapillaris. Evidence for a trophic paracrine relation. Am J Pathol 1999155421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim I, Ryan A M, Rohan R.et al Constitutive expression of VEGF, VEGFR‐1, and VEGFR‐2 in normal eyes. Invest Ophthalmol Vis Sci 1999402115–2121. [PubMed] [Google Scholar]
- 41.Ozaki H, Seo M S, Ozaki K.et al Blockade of vascular endothelial cell growth factor receptor signaling is sufficient to completely prevent retinal neovascularization. Am J Pathol 2000156697–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41a. Rosenfeld P, Brown D M, Heier J S.et al Ranibizumab for neovascular age‐related macular degeneration. N Engl J Med 20063551419–1431. [DOI] [PubMed] [Google Scholar]
- 41b. Brown D M, Kaiser P K, Michels M.et al Ranibizumab versus verteporfin for neovascular age‐related macular degeneration. N Engl J Med 20063551432–1444. [DOI] [PubMed] [Google Scholar]
- 42.Gragoudas E S, Adamis A P, Cunningham E T., Jret al Pegaptanib for neovascular age‐related macular degeneration. N Engl J Med 20043512805–2816. [DOI] [PubMed] [Google Scholar]
- 43.Rakic J M, Lambert V, Devy L.et al Placental growth factor, a member of the VEGF family, contributes to the development of choroidal neovascularization. Invest Ophthalmol Vis Sci 2003443186–3193. [DOI] [PubMed] [Google Scholar]
- 44.Aiello L P, Avery R L, Arrigg P G.et al Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 19943311480–1487. [DOI] [PubMed] [Google Scholar]
- 45.Boyd S R, Zachary I, Chakravarthy U.et al Correlation of increased vascular endothelial growth factor with neovascularization and permeability in ischemic central vein occlusion. Arch Ophthalmol 20021201644–1650. [DOI] [PubMed] [Google Scholar]
- 46.Noma H, Minamoto A, Funatsu H.et al Intravitreal levels of vascular endothelial growth factor and interleukin‐6 are correlated with macular edema in branch retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol 2006244309–315. [DOI] [PubMed] [Google Scholar]
- 47.Kvanta A, Algvere P V, Berglin L.et al Subfoveal fibrovascular membranes in age‐related macular degeneration express vascular endothelial growth factor. Invest Ophthalmol Vis Sci 1996371929–1934. [PubMed] [Google Scholar]
- 48.Adamis A P, Miller J W, Bernal M T.et al Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol 1994118445–450. [DOI] [PubMed] [Google Scholar]
- 49.Usui T, Ishida S, Yamashiro K.et al VEGF164(165) as the pathological isoform: differential leukocyte and endothelial responses through VEGFR1 and VEGFR2. Invest Ophthalmol Vis Sci 200445368–374. [DOI] [PubMed] [Google Scholar]
- 50.Ishida S, Usui T, Yamashiro K.et al VEGF164 is proinflammatory in the diabetic retina. Invest Ophthalmol Vis Sci 2003442155–2162. [DOI] [PubMed] [Google Scholar]
- 51.Shima D T, Gougos A, Miller J W.et al Cloning and mRNA expression of vascular endothelial growth factor in ischemic retinas of Macaca fascicularis. Invest Ophthalmol Vis Sci 1996371334–1340. [PubMed] [Google Scholar]
- 52.Akiyama H, Mohamedali K, Lima‐Silva R.et al Vascular targeting of ocular neovascularization with a VEGF121/gelonin chimeric protein. Mol Pharmacol 2005681543–1550. [DOI] [PubMed] [Google Scholar]
- 53.Avery R L, Pieramici D J, Rabena M D.et al Intravitreal bevacizumab (Avastin) for neovascular age‐related macular degeneration. Ophthalmology 2006113363–372. [DOI] [PubMed] [Google Scholar]
- 54.Rosenfeld P J, Moshfeghi A A, Puliafito C A. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin®) for neovascular age‐related macular degeneration. Ophthalmic Surg Lasers Imaging 200536331–335. [PubMed] [Google Scholar]
- 55.Konner J, Dupont J. Use of soluble recombinant decoy receptor vascular endothelial growth factor trap (VEGF trap) to inhibit vascular endothelial growth factor activity. Clin Colorectal Cancer 20044(Suppl 2)S81–S85. [DOI] [PubMed] [Google Scholar]
- 56.Maier P, Unsoeld A S, Junker B.et al Intravitreal injection of specific receptor tyrosine kinase inhibitor PTK787/ZK222 584 improves ischemia‐induced retinopathy in mice. Graefe's Arch Clin Exp Ophthalmol 2005243593–600. [DOI] [PubMed] [Google Scholar]
- 57.Reich S J, Fosnot J, Kuroki A.et al Small interfering RNA (siRNA) targeting VEGF effectively inhibits ocular neovascularization in a mouse model. Mol Vis 20039210–216. [PubMed] [Google Scholar]
- 58.Gonzales C R. Enhanced efficacy associated with early treatment of neovascular age‐related macular degeneration with pegaptanib sodium: an exploratory analysis. Retina 200525815–827. [DOI] [PubMed] [Google Scholar]
- 59.Cunningham E T, Jr, Adamis A P, Altaweel M.et al A phase II randomized double‐masked trial of pegaptanib, an anti‐vascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmology 20051121747–1757. [DOI] [PubMed] [Google Scholar]
- 60.Hurwitz H I, Fehrenbacher L, Hainsworth J D.et al Bevacizumab in combination with fluorouracil and leucovorin: an active regimen for first‐line metastatic colorectal cancer. J Clin Oncol 2005233502–3508. [DOI] [PubMed] [Google Scholar]
- 61.Miller K D, Chap L I, Holmes F A.et al Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol 200523792–799. [DOI] [PubMed] [Google Scholar]
- 62.Hurwitz H, Fehrenbacher L, Novotny W.et al Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 20043502335–2342. [DOI] [PubMed] [Google Scholar]
- 63.Michels S, Rosenfeld P J, Puliafito C A.et al Systemic bevacizumab (Avastin) therapy for neovascular age‐related macular degeneration twelve‐week results of an uncontrolled open‐label clinical study. Ophthalmology 20051121035–1047. [DOI] [PubMed] [Google Scholar]
- 64.Nguyen Q D, Shah S, Tatlipinar S.et al Bevacizumab suppresses choroidal neovascularisation caused by pathological myopia. Br J Ophthalmol 2005891368–1370. [PMC free article] [PubMed] [Google Scholar]
- 65.Gaudreault J, Fei D, Rusit J.et al Preclinical pharmacokinetics of ranibizumab (rhuFabV2) after a single intravitreal administration. Invest Ophthalmol Vis Sci 200546726–733. [DOI] [PubMed] [Google Scholar]
- 66.Haughney P C, Lowe J, Kearnset al Clinical pharmacokinetics of ranibizumab (Lucentis™) in subjects with AMD. Presented at 2005 ARVO Annual Meeting,Fort Lauderdale, FL, USA. May 20051–5.
- 67.Rosenfeld P J, Schwartz S D, Blumenkranz M S.et al Maximum tolerated dose of a humanized anti‐vascular endothelial growth factor antibody fragment for treating neovascular age‐related macular degeneration. Ophthalmology 20051121048–1053. [DOI] [PubMed] [Google Scholar]
- 68.Siddiqui M A, Keating G M. Pegaptanib: in exudative age‐related macular degeneration. Drugs 2005651571–1577. [DOI] [PubMed] [Google Scholar]
- 69.Hudson P J, Souriau C. Engineered antibodies. Nat Med 20039129–134. [DOI] [PubMed] [Google Scholar]
- 70.Rosenfeld P J, Fung A E, Puliafito C A. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for macular edema from central retinal vein occlusion. Ophthalmic Surg Lasers Imaging 200536336–339. [PubMed] [Google Scholar]
- 71.Fung A E, Rosenfeld P J, Reichel E. The International Intravitreal Bevacizumab Safety Survey: using the internet to assess drug safety worldwide. Br J Ophthalmol 2006901344–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]