Abstract
Aim
To determine whether topical antifungal prophylaxis distributed by paid village health workers (VHWs) in south India is necessary after corneal abrasion to prevent fungal keratitis in a population where half of the ulcers are fungal.
Methods
Two panchayaths (village administrative units in Madurai district with a combined population of 48 039 were followed prospectively for 18 months by 15 VHWs who were trained to identify post‐traumatic corneal abrasions. Patients fulfilling the eligibility criteria were randomised into two groups and treated with either 1% chloramphenicol and 1% clotrimazole ointment or 1% chloramphenicol and a placebo ointment three times a day for 3 days. Patients, doctors and VHWs were blinded to treatment.
Results
During the 18‐month period, 1365 people reported to VHWs with ocular injuries, of whom 374 with corneal abrasions were eligible for treatment. Of these, 368 (98.5%) abrasions healed without complications. Two patients had mild localised allergic reactions to the ointment, two dropped out and two patients in the placebo group developed microscopic culture‐negative corneal stromal infiltrates that healed in 1 week with natamycin drops.
Conclusions
Both fungal and bacterial ulcers that occur after traumatic corneal abrasions seem to be effectively prevented in a village setting using only antibiotic prophylaxis.
With the worldwide decrease in trachoma and other traditional causes of blindness, such as onchocerciasis and leprosy, the World Health Organization (WHO) has recognised that corneal blindness resulting from microbial keratitis is emerging as an important cause of visual disability.1 The incidence of corneal ulcers in south‐east Asia is especially high compared with other parts of the world. A retrospective study from south India2 in 1993 found an incidence of 113 ulcers per 100 000 annually, 10 times that of the US,3 whereas in Nepal4 the incidence was 799 per 100 000. Similar high rates of corneal ulceration have been reported from Bhutan5 and Burma,6 leading to the warning that a “silent epidemic” of corneal blindness may be occurring in this region as a result of these infections.7
Studies in south‐east Asia have also shown that the greatest risk factor for corneal ulceration is corneal abrasion. In Nepal8 and India,9 only 53% and 65%, respectively, of all patients with corneal ulcers could remember having a prior abrasion, but these percentages are probably low because of recall bias. In Bhutan,5 where 98% of ulcers are bacterial, no ulceration developed in any patients treated with 1% chloramphenicol ointment after corneal abrasion. Meanwhile, the incidence of ulceration in the surrounding control districts remained at 301 per 100 000. From these and other observations, it seems that trivial corneal injury is indeed the main risk factor for microbial keratitis in developing countries and that proper prophylactic treatment of these injuries can prevent ulcers from occurring. In the Bhaktapur Eye Study in Nepal,4 keratitis was prevented in 407 (96%) of the 424 patients reporting within 48 h after corneal abrasion. However, there were 18 “breakthrough” ulcers that occurred despite prophylaxis with 1% chloramphenicol ointment, and all were culture‐proved bacterial infections. This was unexpected because it is known that 20% of culture‐proved ulcers in Nepal8 are fungal, and therefore at least a few of the breakthrough ulcers should have grown fungal pathogens. In a recent study in Burma,10 where two thirds of all corneal ulcers are caused by fungi, microbial keratitis was found to be prevented in all patients presenting with a corneal abrasion, using a prophylactic combination of 1% chloramphenicol and 1% clotrimazole ointment for 3 days. It was assumed that clotrimazole was effective in preventing fungal ulcers, whereas chloramphenicol prevented bacterial infections, but is this assumption correct? Is an antifungal drug really needed to prevent fungal keratitis after corneal abrasion in a country where fungal pathogens commonly cause ulceration?
In south India, where half of all corneal ulcers are culture‐positive for fungi,9 it is reported anecdotally that treatment of corneal abrasions with an antibiotic ointment invariably prevents subsequent corneal ulceration from all causes. This observation, along with the curious lack of breakthrough fungal ulcers in the Bhaktapur Eye Study, was the genesis of this placebo‐controlled study in south India, which was conducted to determine whether antifungal prophylaxis is necessary after corneal abrasion to prevent fungal keratitis. The second goal of this study was to determine whether or not paid village health workers (VHWs) are capable of preventing corneal ulceration in a defined population.
Methods
For the purpose of the study, two panchayaths (village administrative units) with populations of 25 000 each, Sholavandan and Melur, were chosen in Madurai district. The combined population of 48 039 was followed for a period of 18 months (September 2002 to March 2004) by 15 paid VHWs whose qualifications were a high school education and fluency in English. The VHWs were trained at the Aravind Eye Hospital to identify corneal abrasions using fluorescein strips and a blue torch, and were taught to measure visual acuity. Supplies, including sterile single‐packed fluorescein strips, a pictorial study manual, a blue torch and a laminated “E” chart, were provided to each worker. The two panchayaths were segregated into groups of villages that were allotted to the VHWs based on proximity to the worker's residence. Each VHW was responsible for keeping track of all ocular injuries occurring in a defined population of 3500–4000 people. The demographic details of the residents of the panchayaths were collected in a door‐to‐door survey by the VHWs between July and August 2002, and the total population was enumerated. Two referral centres provided support for the VHWs, the Sholavandan Panchayath Study Center and the Aravind Eye Hospital, Madurai, Tamil Nadu, India, which was in close proximity to Melur. Each centre was staffed by resident ophthalmologists and ophthalmic assistants who were available daily to see the study patients. Before beginning recruitment of patients, a pilot study was carried out in the study areas for 1 month, and the procedures and treatment regimen were followed according to the protocol.
Patients
Inclusion criteria
Participants were included in the study if they
were residents of the study area;
had corneal abrasion after ocular injury; confirmed by clinical examination with fluorescein stain and a blue torch;
reported within 48 h of the injury;
were patients aged >5 years of age.
Exclusion criteria
Patients were excluded from the study if they
were not residents of the study area;
had clinically evident corneal infection;
had penetrating corneal injury or stromal laceration;
had bilateral ocular trauma;
had pre‐existing blindness (<6/60) in the non‐traumatised eye;
had initiation of topical or systemic antibiotic treatment before examination by study personnel;
had incomplete lid closure;
had diabetes;
had other injuries requiring hospitalisation;
had trichiasis;
had dacryosystitis
were unwilling to participate.
Treatment
The VHWs accompanied all eligible patients to one of the two referral centres, where the diagnosis of a corneal abrasion was confirmed by an ophthalmologist. After obtaining written consent, patients were randomised by computer‐generated numbers into two treatment groups, and each patient was given two tubes of ointment that were labelled either A or B. Ophthalmologists, VHWs and patients were blinded to the contents of the tubes. Eligible patients were treated immediately with an application of each ointment, and instructed to apply both ointments two more times the first day and three times daily for the next 2 days, making a total of nine applications. House visits were made during the 3‐day period by the VHWs to check for compliance. On the third day, the participants were brought to the referral centre by the VHWs to be examined by an ophthalmologist at the slit lamp. Those participants not returning to the referral centre were visited at their homes by the VHWs and examined with a fluorescein strip and a blue torch. The primary outcome was complete epithelialisation of the cornea without evidence of infection, or alternatively, the development of a corneal infiltrate or ulcer at the site of the abrasion. All study drugs (1% chloramphenicol ointment, 1% clotrimazole ointment and placebo ointment) were prepared by the pharmaceutical division at the Aravind Eye Hospital and seemed to be identical in both groups. The biostatistics department at Aravind Eye Hospital was custodian of the treatment code. The study was approved by the institutional review board of the Aravind Eye Care system and the WHO South East Asia Regional Office in Delhi.
Results
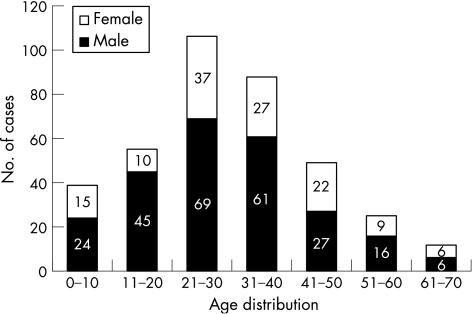
During the 18‐month period (October 2002–March 2004), 1365 ocular injuries were reported and identified by the VHWs. Of these, 409 (30%) patients had corneal abrasions, 334 (24.5%) had conjunctival lacerations, 91 (6.7%) had corneal injuries other than abrasions and 8 (0.59%) presented with corneal ulcers (table 1). Thirty five patients with corneal abrasion were excluded from the study because of the exclusion criteria, and of the remaining 374, 123 (32.9%) reported in the first 12 h, 89 (23.8%) from 13 to 18 h, 94 (25.1%) from 19 to 24 h and 68 (18.2%) from 25 to 48 h (table 2). Most abrasions occurred in the 21–40‐year‐old group (51.9%), and more male patients (66.3%) than female patients (33.7%) were diagnosed with abrasions (fig 1).
Table 1 Ocular trauma in a defined population of 48 039 during 18 months.
| Clinical findings | No of cases* | Annual incidence /100 000 population |
|---|---|---|
| Corneal abrasion | 409 | 568 |
| Corneal ulcer | 8 | 11 |
| Conjunctival injury | 334 | 464 |
| Corneal injury | 91 | 126 |
| Lid injury | 41 | 57 |
| Other injury | 31 | 43 |
| No clinical findings | 164 | 228 |
| Reported only | 287 | 398 |
| Total | 1365 | 1894 |
*In a population of 48 039 followed for 18 months.
Table 2 Time interval between corneal abrasion and initiation of prophylactic treatment in 374 patients.
| Hours elapsed | Frequency (%) |
|---|---|
| 0–6 | 107 (28.6) |
| 7–12 | 16 (4.3) |
| 13–18 | 89 (23.8) |
| 19–24 | 94 (25.1) |
| 25–48 | 68 (18.2) |
| Total | 374 (100) |
Figure 1 Age and sex distribution of 374 patients treated for corneal abrasions.
The 374 eligible patients with abrasions were enrolled and 205 (54.8%) were randomised to treatment A and 169 (45.2%) to treatment B. Four of the patients had adverse events, two dropped out of the study for unspecified personal reasons and 368 (98.5%) healed without complications. When the codes for treatment A (1% chloramphenicol ointment and placebo ointment) and treatment B (1% chloramphenicol ointment and 1% clotrimazole ointment) were unblinded, the four patients with adverse reactions were all in the treatment A (placebo) group. Two of the patients had mild chemosis and irritation secondary to the antibiotic and placebo ointments that were applied, and two of the patients developed small single corneal infiltrates at the site of the abrasion measuring <0.5 mm in diameter at the slit lamp and located in the anterior stoma just beneath Bowman's membrane. Both infiltrates, when cultured, were negative for fungi, but were treated empirically with 5% natamycin drops every 2 h for 1 week and resolved completely without complications.
Discussion
This study is the third of a multicentre project carried out in three countries in South East Asia (Bhutan, Burma and India) in collaboration with WHO in New Delhi and the Aravind Eye Hospital in Madurai. In this population‐based, placebo‐controlled, prospective, double‐blinded clinical treatment trial, a population of 48 039 living in the Sholavandan and Melur panchayaths of Madurai district was kept under daily surveillance for a period of 18 months from September 2002 to March 2004. All patients with corneal abrasions who met the eligibility criteria were randomised and treated with topical chloramphenicol and clotrimazole, or topical chloramphenicol and a placebo ointment, to determine whether or not antifungal prophylaxis is necessary for the prevention of corneal ulcers in this population in which 50% of all ulcers are positive for fungi. The surprising primary outcome of the study was that an antifungal prophylaxis is apparently not necessary to prevent fungal keratitis. This becomes even more interesting when we consider that 47% of all the fungal ulcers in south India are caused by Fusarium spp,9 arguably one of the most destructive and invasive of all corneal pathogens.
These results are difficult to explain. There are examples of some antibiotics having antifungal activity. Fusarium oxysporum keratitis has been reported to respond to treatment with tobramycin,11 and in vitro studies have shown antibiotic inhibition of pectolytic and cellulolytic enzyme activity in F oxysporum by amoxycillin, and to a lesser extent by chloramphenicol, erythromycin and raficillin.12 In the outbreak of Fusarium keratitis associated with contact lens wear that was recently reported from Singapore,13 11 (16.2%) of the 68 eyes treated with topical antibiotics alone (gentamicin and cefalzolin) resolved “without the need for specific antifungal therapy”. Fusarium spp was isolated from all 11 eyes. A more pedestrian explanation, however, for why we did not see fungal ulcers in our study, even in the placebo group, is that our long‐held belief that fungal organisms are inoculated into the corneal stoma at the time of the corneal abrasion is incorrect. This is reinforced by data from India9 showing that abrasions caused by organic materials are just as likely to develop bacterial as fungal ulcers. Fungal pathogens may only be opportunists, waiting in the environment for a slow‐healing epithelial abrasion to provide access to the corneal stroma. Perhaps rapid epithelialisation combined with a modest antifungal effect from the antibiotic, or from the ointment base itself, is enough to discourage corneal infection. The fact remains that at an incidence of 113 per 100 000, we expected 80 corneal ulcers, half of them fungal, to occur in our study population of 48 039 over 18 months. Instead, only eight “non‐abrasion associated” corneal ulcers were seen (11/100 000), and 368 (98.5%) of the 374 abrasions receiving prophylactic treatment healed without complications. In the placebo treatment group, where we expected to see 20 breakthrough fungal ulcers, we found only two patients with culture‐negative microscopic stromal infiltrates that we presumed were fungal. By contrast, a large nearby “control” village of 3094 people had five ulcers in 1 year, an incidence of 161 per 100 000.
Secondary outcomes of the study included a determination of the incidence of ocular trauma in the population (table 1), which was comparable to the data reported from Nepal.4 Unlike the studies in Nepal,4 Bhutan5 and Burma,10 patients with corneal abrasions in south India reported for treatment over a longer period (table 2), but this did not seem to affect the efficacy of the ulcer prevention regimen. In addition, paid non‐governmental VHWs hired locally in India were found to be as effective in implementing and sustaining a corneal ulcer prevention programme as were volunteer VHWs in Bhutan5 and governmental VHWs in Burma.10 As in those countries, the compliance of the Indian population was directly related to the local involvement of the VHWs.
These findings raise an important question. We have shown previously that microbial keratitis after corneal abrasion can be prevented at the village level by simple public health strategies using antibiotic and antifungal prophylaxis tailored to the prevalence of pathogens causing corneal ulcers in the population.10 This study, however, raises the key question of which therapeutic regimen is actually most cost‐effective in achieving this goal. At present, another trial with a larger population is being conducted in south India to determine whether antibiotics alone can indeed prevent both fungal and bacterial keratitis after corneal abrasion. Hopefully the question will be definitely answered.
Acknowledgements
Consultative support was provided by World Health Organization Collaborating Centers in San Francisco, New Delhi and Madurai.
Abbreviations
VHWs - village health workers
Footnotes
This study was supported by a grant from the World Health Organization/South East Asia Regional Office in New Delhi, and by material, logistical, technical and human resources from the Aravind Medical Research Foundation, Aravind Eye Care System and Lions Aravind Institute of Community Ophthalmology in Madurai.
Competing interests: None declared.
References
- 1.Resnikoff S, Pascolini D, Elya'ale D.et al Global data on visual impairment in the year 2002. Bull World Health Org 200482844–855. [PMC free article] [PubMed] [Google Scholar]
- 2.Gonzales C A, Srinivasan M, Whitcher J P.et al Incidence of corneal ulceration in Madurai District, South India. Ophthalmol Epidemiol 19963159–166. [DOI] [PubMed] [Google Scholar]
- 3.Erie J C, Nevitt M P, Hodge D O.et al Incidence of ulcerative keratitis in a defined population from 1950–1988. Arch Ophthalmol 19931111665–1671. [DOI] [PubMed] [Google Scholar]
- 4.Upadhyay M P, Karmacharya P C, Koirala S.et al The Bhaktapur Eye Study: ocular trauma and antibiotic prophylaxis for the prevention of corneal ulceration in Nepal.Br J Ophthalmol 200185388–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Getshen K, Srinivasan M, Upadhyay M P.et al Corneal ulceration in South East Asia. I: A model for the prevention of bacterial ulcers at the village level in rural Bhutan, Br J Ophthalmol 200690276–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization Guidelines for the management of corneal ulcer at primary, secondary, and tertiary care health facilities in the South‐East Asia region. SEA/Opthal/126. World Health Organization Regional Office for South‐East Asia 20041–3.
- 7.Whitcher J, Srinivasan M. Corneal ulceration in the developing world ‐ a silent epidemic. Br J Ophthalmol 199781622–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Upadhyay M D, Karmacharya P C, Koirala S.et al Epidemiologic characteristics, predisposing factors, and etiologic diagnosis of corneal ulceration in Nepal. Am J Ophthalmol 19911192–99. [DOI] [PubMed] [Google Scholar]
- 9.Srinivasan M, Gonzales C A, George C.et al Epidemiology and etiological diagnosis of corneal ulceration in Madurai, South India. Br J Ophthalmol 19978965–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maung N, Thant C C, Srinivasan M.et al Corneal ulceration in South East Asia II: a strategy for the prevention of fungal keratitis at the village level in Myanmar. Br J Ophthalmol 200690968–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chodosh J, Miller D, Tu E.et al Tobramycin‐responsive Fusarium oxysporum keratitis. Can J Ophthalmol 20003529–30. [DOI] [PubMed] [Google Scholar]
- 12.Mehta A, Chopra S, Mehta P. Antibiotic inhibition of pectolytic and cellulolytic enzyme activity in two Fusarium species. Mycopathologia 1993124185–188. [DOI] [PubMed] [Google Scholar]
- 13.Khor W, Aung T, Saw S.et al An outbreak of Fusarium keratitis associated with contact lens wear in Singapore. JAMA 20062952867–2873. [DOI] [PubMed] [Google Scholar]