Abstract
Objective
To investigate retrobulbar haemodynamics in patients with cataract.
Subjects and methods
Colour Doppler imaging of the ophthalmic artery was carried out on the eye scheduled for surgery in 30 patients with cataract and in one randomly selected eye of 100 healthy controls. The peak systolic velocity, mean velocity, end diastolic velocity and resistivity index in the ophthalmic artery were computed and adjusted for the influence of age and mean arterial pressure. Cataract type was recorded and lens opacity was measured with an opacity lensmeter. Odds ratio (OR) for cataract was analysed in a logistic regression model, depending on the adjusted blood‐flow parameters, age and smoking status.
Results
The mean (SD) age was 45.5 (17.7) and 67.6 (5.8) years in controls and patients with cataract, respectively (p<0.001). The female to male ratio was 54:46 and 13:17, respectively (p = 0.41). Significant predictors of cataract in a forward stepwise logistic regression analysis were age (OR = 1.194; 95% confidence interval (CI) = 1.103 to 1.292; p<0.001), smoking status (OR = 14.119; 95% CI = 2.753 to 72.398; p = 0.002) and mean blood‐flow velocity in the ophthalmic artery (OR = 0.731; 95% CI = 0.607 to 0.881; p = 0.001). Adjusted mean velocity was significantly lower in patients with cataract, even when only age‐matched (age >55 years) non‐smokers (31 controls, 19 patients with cataract) were considered (p = 0.003). Lens opacity and the type of cataract had no influence on the present findings.
Conclusion
High mean velocity in the ophthalmic artery may be associated with a reduced risk of cataract.
Lens opacities have been reported to be important predictors of mortality, with reported risk ratios ranging between 1.5 and 2,1,2,3,4,5 possibly because they are associated with risk factors that also affect mortality.6,7 Although the type of cataracts and the associated risk factors are unclear,3,8 age,8,9 diabetes mellitus,8,10,11 body mass index,12,13 cigarette smoking11,14,15,16 and exposure to radiation or ultraviolet light17,18 have been described as possible contributors to the development of lens opacities. Also, there are indications that people with hypertension may be more likely to have lens opacities,6,19 although this could not be confirmed in all studies.20
The association between cardiovascular risk factors and lens opacity suggests that changes in ocular blood flow may be present in patients with cataract. To date, most studies on ocular blood flow in patients with cataract have only investigated the effect of regional anaesthesia.21,22,23,24,25,26 This pilot study was set to explore possible genuine changes in ocular blood flow in patients with cataract.
Patients and methods
Setting
This prospective study was conducted at the Department of Ophthalmology, University Hospital Basle (Basle, Switzerland) and was approved by the institutional ethics committee. Informed consent was obtained from each patient and control. The research followed the tenets of the Declaration of Helsinki.
Study population
Thirty patients scheduled for cataract surgery as well as 100 healthy controls with no lens opacification in a dilated eye examination were recruited. As a lack of previous studies on this issue precluded a proper sample size calculation during the planning of the study, the number of recruited patients and controls was determined arbitrarily. Furthermore, because we considered that a biological association between cataract and changes in blood flow should be more marked in relatively young patients, we did not apply any age limitations during recruitment.
A complete ophthalmological examination was carried out on all subjects. One eye was randomly selected in controls, and the eye scheduled for cataract surgery was selected in patients with cataract. Patients or controls with ocular conditions that may have affected the colour Doppler imaging (CDI) measurement results, such as glaucoma, age‐related maculopathy and high degree of myopia, and patients or controls with a history of laser treatment or previous intraocular surgery were excluded. Furthermore, patients or controls with a history of systemic hypertension, diabetes mellitus according to the World Health Organization (WHO) criteria (1999)27 and any topical or systemic drugs were excluded from the study. Sex and smoking habits (smoker/non‐smoker) were recorded for all subjects. Patients or controls were classified as smokers when a current or ever‐smoking history was reported.
Ocular blood‐flow velocity measurement
Blood‐flow velocity in the ophthalmic artery was measured with a Siemens Quantum 2000 (Siemens Albis AG, Zurich, Switzerland) using a 7.5‐MHz linear phase‐array transducer. The transducer was applied gently to the closed eyelid using a coupling gel, and care was taken to avoid applying any pressure to the eye. During the examination, patients were in a supine position, with the upper torso tilted forward at an angle of about 30°. The ophthalmic artery was traced nasal to the optic nerve after their crossing, and measurements were taken approximately 10–15 mm posterior to the globe. The proximal and distal portions of the vessel were imaged as well as possible to determine the Doppler‐flow angle and apply proper corrections. All the CDI measurements were taken by BD, who was blinded to the diagnosis. During the entire examination, systolic arterial pressure (SAP), diastolic arterial pressure (DAP) and heart rate were determined by an automatic blood pressure apparatus every 5 min. These measurements were taken from the arm opposite to the study eye. The mean arterial pressure (MAP) in the brachial artery was calculated according to the formula MAP = DAP+1/3 (SAP−DAP) and the values obtained were averaged for the examination time.
The peak systolic velocity (PSV) was defined as the highest velocity of blood flow during the systolic phase of the cardiac cycle, and the end diastolic velocity (EDV) as the velocity of blood flow at the end of the diastolic phase of the cardiac cycle. The mean velocity, defined as the average velocity of blood flow throughout the cardiac cycle, and the resistivity index (RI = (PSV−EDV)/PSV) were computed in every patient and control.
Measurement of lens opacity
The opacity lensmeter (OLM) 701 (Interzeag, Schlieren, Switzerland)28 was used to quantify lens opacity in the group of patients with cataract. The mean value of five consecutive measurements was used for statistical analysis. The technical principle has been described previously.28 To produce stray light in the lens of the patient, the instrument emits a modulated dark‐red light beam, which runs along the optic axis of the lens. The modulation of the light source renders the measurement insensitive to the surrounding light. The stray light, but not the reflected light, is detected by the instrument and converted into an electrical impulse, the value of which is expressed in a numerical scale.
Statistical analysis
Data were extracted from prospectively completed forms and analysed using SPSS V.11.5. Sex distribution in the two groups was compared by means of Fisher's exact test. Blood‐flow velocity parameters in the ophthalmic artery were adjusted for the influence of age29,30 and systemic MAP by regressing each parameter on age and systemic MAP in a multiple regression analysis. The CDI readings were taken only from the ophthalmic artery, because the large number of variables obtained from all the retrobulbar vessels would have precluded any meaningful statistical analysis with the chosen sample size. Furthermore, the ophthalmic artery allows measurement with a high reproducibility.31 The mean values of the adjusted variables were compared using the t test. In addition, the correlation of ocular blood‐flow velocity and cataracts was scrutinised in a logistic regression analysis. A forward stepwise logistic regression model was chosen for this exploratory design. As predicting variables, retrobulbar blood‐flow velocity parameters (PSV, EDV, mean velocity and resistivity index for the ophthalmic artery) adjusted for age and systemic MAP, age, systemic MAP, and smoking status were computed. The forward stepwise computation retained predicting variables in the final model on the basis of the significance of the change in log likelihood ratio (p value for removal >0.1). Odds ratios (ORs) and the corresponding 95% confidence interval (CI) were estimated.
Results
Table 1 lists the demographic data of the study population. Patients with cataract were significantly older (p<0.001). Sex distribution (p = 0.41) and smoking habits (p = 0.34) were comparable between the study groups. The recruited patients with cataract showed nuclear (n = 17), cortical (n = 7) or posterior subcapsular cataracts (n = 6). The OLM values (mean (SD)) of the patients with nuclear cataract (47.1 (15.5) U) were significantly higher than those among patients with cortical (27.3 (5.5) U) or subcapsular (36.7 (18.5) U) cataract (one‐way analysis of variance p = 0.02). SAP and MAP were significantly higher in the cataract group (p = 0.008 and 0.003, respectively). The differences in diastolic blood pressure and heart rate were not significant.
Table 1 Demographic data of controls and the cataract group (mean (SD)).
| Controls (n = 100) | Cataract group (n = 30) | p Value | |
|---|---|---|---|
| Age (years) | 45.5 (17.7) | 67.6 (5.8) | <0.001 |
| Sex (female/male) | 54/46 | 13/17 | 0.41 |
| Smoker | 23 | 10 | 0.34 |
| Systolic BP (mm Hg) | 132.7 (20.0) | 142.9 (17.2) | 0.008 |
| Mean BP (mm Hg) | 96.2 (15.4) | 104.8 (12.7) | 0.003 |
| Diastolic BP (mm Hg) | 75.4 (12.0) | 78.4 (10.4) | 0.18 |
| Heart rate (beats/min) | 69.5 (9.6) | 72.3 (11.1) | 0.22 |
BP, blood pressure.
Table 2 lists the retrobulbar blood‐flow velocity parameters. PSV, EDV and mean velocity were lower in the group of patients with cataract, whereas mean resistivity index was comparable to that in controls. After adjusting each parameter for age and systemic MAP in a multiple regression analysis, only mean velocity was found to be significantly different between the two groups.
Table 2 Blood‐flow parameters in the ophthalmic artery in controls and in the cataract group (mean (SD)).
| Controls (n = 100) | Cataract group (n = 30) | p Value | |
|---|---|---|---|
| PSV (cm/s) | 40.5 (7) | 35.6 (9.9) | 0.016 |
| EDV (cm/s) | 9.5 (2.8) | 7.4 (2.9) | 0.002 |
| MV (cm/s) | 17.5 (4.8) | 13.7 (4) | <0.001 |
| RI | 0.76 (0.05) | 0.78 (0.07) | 0.21 |
| Adjusted PSV (cm/s) | 39.9 (6.8) | 37.6 (9.9) | 0.22 |
| Adjusted EDV (cm/s) | 9.3 (2.7) | 8.2 (2.9) | 0.08 |
| Adjusted MV (cm/s) | 17.3 (4.8) | 14.6 (4.1) | 0.004 |
| Adjusted RI | 0.76 (0.05) | 0.77 (0.07) | 0.42 |
EDV, end diastolic velocity; MV, mean velocity; PSV, peak systolic velocity; RI, resistivity index.
Univariate logistic regression analysis of the haemodynamic parameters for the diagnosis of cataracts disclosed a significant influence of the mean velocity adjusted for age and systemic MAP (OR 0.87, CI 0.78 to 0.96, p = 0.008), but not for PSV (OR 0.96, CI 0.90 to 1.0, p = 0.14), EDV (OR 0.86, CI 0.73 to 1.0, p = 0.07) or resistivity index (OR 40.43, CI 0.022 to 73500.2, p = 0.33). In the forward stepwise approach, age, smoking and mean velocity were found to be significant contributors to the final model (table 3).
Table 3 Significant contributors to the logistic regression model.
| OR | 95% CI | p Value | |
|---|---|---|---|
| Age | 1.194 | 1.103 to 1.292 | <0.001 |
| Smoking | 14.119 | 2.753 to 72.398 | 0.002 |
| OA‐MV | 0.731 | 0.607 to 0.881 | 0.001 |
OA‐MV, mean blood‐flow velocity of the ophthalmic artery.
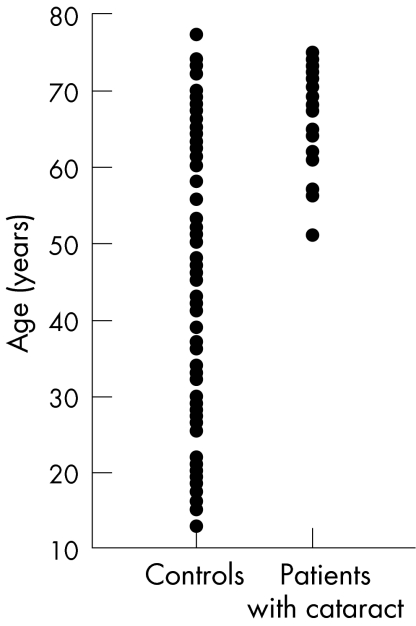
Although a clear overlap between the groups existed for age, most of the controls were younger than the patients with cataract (fig 1), which could have influenced the results. Therefore, a cut‐off value of 55 years of age (mean (SD) age 66.3 (5.1) and 68.1 (5.0) years in controls and patients with cataract, respectively; p = 0.16) was applied and a retrospective analysis was carried out for this reduced sample of 29 patients with cataract and 33 controls. In the multivariate logistic regression analysis, the risk profile for cataracts disclosed again a significant influence of the adjusted mean velocity (p = 0.004), but not that of age (p = 0.09) or of smoking (p = 0.74). The interaction between mean velocity and smoking in the model was not significant either (p = 0.30). However, only two of the controls and 10 of the patients with cataract were smokers, whereas 31 of the controls and 19 of the patients with cataract were non‐smokers. When only non‐smokers of the age‐matched groups were considered, adjusted mean velocity for age and blood pressure (mean (SD) blood velocity 17.0 (4.3) and 13.1 (2.8) cm/s in controls and patients with cataract, respectively) remained a significant contributor to cataract (p = 0.003). Owing to the small number of smokers among controls (n = 2), a statistical analysis among smokers was not meaningful.
Figure 1 Age distribution of controls and patients with cataract.
Furthermore, the influence of the type of cataract and the grade of lens opacity measured with the OLM was scrutinised among patients with cataract. The mean (SD) adjusted mean velocity in patients with nuclear (14.4 (3.24) cm/s), cortical (14.7 (3.2) cm/s) and posterior (14.6 (7.1) cm/s) subcapsular cataracts was statistically comparable (one‐way analysis of variance p = 0.97), and no correlation between adjusted mean velocity and the OLM values was found (p = 0.54).
Discussion
This study suggests that ocular haemodynamic parameters may be perturbed in patients with cataracts. The retrobulbar blood‐flow velocity parameters PSV, EDV and mean velocity were lower in the group of patients with cataract. After adjusting for age and systemic MAP, the mean velocity was still significantly lower in patients with cataract, whereas PSV and EDV were not. However, PSV and EDV describe unique time points during the cardiac cycle, whereas mean velocity represents an average value for the entire cardiac cycle, and is, therefore, possibly more directly related to bulk blood flow. Logistic regression analysis also suggested a significant influence of the mean velocity, adjusted for age and MAP. Owing to the large difference in age between the two groups and despite a good overlap at the higher end of the spectrum and age adjustment of the blood‐flow parameters, a retrospective analysis was carried out in subsets of the original study population, showing that even in age‐matched patients with cataract and also when only non‐smokers were considered, patients with cataract had a lower mean velocity of blood flow in the ophthalmic artery. However, no difference in mean velocity was found among the cataract subgroups. The number of patients in the subgroups was probably too small to detect any differences. In addition, there was no association between the degree of lens opacification and the mean velocity of the ophthalmic artery, suggesting no direct inter‐relation between the severity of lens opacity and altered ocular blood flow.
It is tempting to interpret the relationship found between altered blood‐flow velocity and cataracts in this study as being related to vascular pathology, which is observed more often in patients developing cataracts.32 Indeed, a nationwide case–control study33 of patients undergoing cataract surgery compared with age‐matched controls also found some association between visually significant cataracts requiring surgery and atherosclerosis. However, because the prevalence rates of both cataracts and atherosclerosis increase with age, a biological association between cataracts and atherosclerosis should be inferred only based on relatively young patients (aged between 65 and 69 years).33 Evidence allowing conjectures regarding a direct pathophysiological relationship between cataract and atherosclerosis is relatively scarce and, although the mean age of patients with cataract in our study was 66 years, possibly suggesting a pathophysiological relationship between cataracts and atherosclerosis, the present findings should be interpreted carefully. Importantly, as CDI measurements of only blood‐flow velocity and not blood‐volume flow, and because assessment of blood flow, or more generally of haemodynamics, is not limited to the consideration of quantitative aspects, the present results can only suggest a different quality of ocular blood flow in patients with cataract. Altered blood flow may contribute to inadequate supply of metabolic nutrients to the lens. Alternatively, vascular dysregulation with reperfusion may be a source of free radicals, as has been hypothesised for glaucoma,34 in particular because oxidation is a hallmark of age‐related nuclear cataract.35 In animal studies, antioxidants seem to be effective in attenuating and delaying the onset of cataract development (eg, diabetic cataracts).36 However, whether a reduced oxygen delivery through impaired blood flow plays a part in the formation of a specific type of cataract remains to be elucidated.
In this study, besides ocular blood‐flow parameters, which were adjusted for age and systemic MAP, age was found to be a significant contributor to cataract formation. This finding is not surprising,8,9,16 and is probably related to the duration of exposure to factors such as ultraviolet radiation and oxidative stress, which may lead to lens opacification.18,37,38,39,40 In an earlier study,41 age‐specific mean systemic blood pressure was found to be slightly higher in patients with cataract than in non‐patients with cataract. The findings in this study were similar. However, in contrast with the age factor, arterial blood pressure did not seem to have an effect independent of ocular blood‐flow velocity, as shown by logistic regression analysis, or was too small to be unveiled with a small number of patients. As there was a significant difference in the mean velocity between controls and patients with cataract in a retrospective analysis of a subset of subjects aged >55 years, it can be inferred that the finding of a different ocular blood flow in patients with cataract was not a statistically significant artefact.
Smoking also had a statistically significant contribution to the present logistic regression model, but the CI for the OR was very large, owing to the small number of smokers among controls. In previous cross‐sectional15 and differently designed studies,13,42,43,44 smoking has been directly related to nuclear cataract, possibly being dose dependent. Although several studies have shown a dose–response relationship between the cumulative amount of smoking and the risk of nuclear cataracts, the effect of smoking on non‐nuclear cataracts remains controversial.3,19,43,45 Owing to the relatively small number of smokers among controls, the effect of blood flow on various types of cataract could not be analysed among smokers. Furthermore, past and current smoking habits were not distinguished, and the cumulative amount of smoking was not taken into account.
In conclusion, lower local blood‐flow velocity in the ophthalmic artery seems to be associated with the presence of cataracts. Even when age‐matched groups are considered and smokers are excluded, patients with cataract yield lower ocular blood‐flow parameters.
Abbreviations
CDI - colour Doppler imaging
DAP - diastolic arterial pressure
EDV - end‐diastolic velocity
MAP - mean arterial pressure
OLM - opacity lensmeter
PSV - peak systolic velocity
SAP - systolic arterial pressure
Footnotes
Funding: The Swiss Federal Scholarship Commission for foreign students supported IK.
Competing interests: None.
References
- 1.Minassian D C, Mehra V, Johnson G J. Mortality and cataract: findings from a population‐based longitudinal study. Bull World Health Organ 199270219–223. [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson J R, Sparrow J M, Gibson J M.et al Cataract and survival in an elderly nondiabetic population. Arch Ophthalmol 1993111675–679. [DOI] [PubMed] [Google Scholar]
- 3.West S K, Munoz B, Istre J.et al Mixed lens opacities and subsequent mortality. Arch Ophthalmol 2000118393–397. [DOI] [PubMed] [Google Scholar]
- 4.Wang J J, Mitchell P, Simpson J M.et al Visual impairment, age‐related cataract, and mortality. Arch Ophthalmol 20011191186–1190. [DOI] [PubMed] [Google Scholar]
- 5.Reidy A, Minassian D C, Desai P.et al Increased mortality in women with cataract: a population based follow up of the North London Eye Study. Br J Ophthalmol 200286424–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein B E, Klein R, Jensen S C.et al Hypertension and lens opacities from the Beaver Dam Eye Study. Am J Ophthalmol 1995119640–646. [DOI] [PubMed] [Google Scholar]
- 7.Borger P H, van Leeuwen R, Hulsman C A.et al Is there a direct association between age‐related eye diseases and mortality? The Rotterdam Study. Ophthalmology 20031101292–1296. [DOI] [PubMed] [Google Scholar]
- 8.Hennis A, Wu S Y, Nemesure B.et al Risk factors for incident cortical and posterior subcapsular lens opacities in the Barbados Eye Studies. Arch Ophthalmol 2004122525–530. [DOI] [PubMed] [Google Scholar]
- 9.Leske M C, Sperduto R D. The epidemiology of senile cataracts: a review. Am J Epidemiol 1983118152–165. [DOI] [PubMed] [Google Scholar]
- 10.Podgor M J, Cassel G H, Kannel W B. Lens changes and survival in a population‐based study. N Engl J Med 19853131438–1444. [DOI] [PubMed] [Google Scholar]
- 11.Harding J J, Egerton M, van Heyningen R.et al Diabetes, glaucoma, sex, and cataract: analysis of combined data from two case control studies. Br J Ophthalmol 1993772–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chatterjee A, Milton R C, Thyle S. Prevalence and aetiology of cataract in Punjab. Br J Ophthalmol 19826635–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nirmalan P K, Robin A L, Katz J.et al Risk factors for age related cataract in a rural population of southern India: the Aravind Comprehensive Eye Study. Br J Ophthalmol 200488989–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hankinson S E, Willett W C, Colditz G A.et al A prospective study of cigarette smoking and risk of cataract surgery in women. JAMA 1992268994–998. [PubMed] [Google Scholar]
- 15.Klein B E, Klein R, Linton K L.et al Cigarette smoking and lens opacities: the Beaver Dam Eye Study. Am J Prev Med 1993927–30. [PubMed] [Google Scholar]
- 16.West S, Munoz B, Schein O D.et al Cigarette smoking and risk for progression of nuclear opacities. Arch Ophthalmol 19951131377–1380. [DOI] [PubMed] [Google Scholar]
- 17.Bochow T W, West S K, Azar A.et al Ultraviolet light exposure and risk of posterior subcapsular cataracts. Arch Ophthalmol 1989107369–372. [DOI] [PubMed] [Google Scholar]
- 18.West S K, Duncan D D, Munoz B.et al Sunlight exposure and risk of lens opacities in a population‐based study: the Salisbury Eye Evaluation project. JAMA 1998280714–718. [DOI] [PubMed] [Google Scholar]
- 19.Chen T T, Hockwin O, Dobbs R.et al Cataract and health status: a case‐control study. Ophthalmic Res 1988201–9. [DOI] [PubMed] [Google Scholar]
- 20.Klein B E, Klein R, Lee K E. Diabetes, cardiovascular disease, selected cardiovascular disease risk factors, and the 5‐year incidence of age‐related cataract and progression of lens opacities: the Beaver Dam Eye Study. Am J Ophthalmol 1998126782–790. [DOI] [PubMed] [Google Scholar]
- 21.Spraul C W, Amann J, Lang G E.et al Effect of cataract extraction with intraocular lens implantation on ocular hemodynamics. J Cataract Refract Surg 1996221091–1096. [DOI] [PubMed] [Google Scholar]
- 22.Findl O, Dallinger S, Menapace R.et al Effects of peribulbar anesthesia on ocular blood flow in patients undergoing cataract surgery. Am J Ophthalmol 1999127645–649. [DOI] [PubMed] [Google Scholar]
- 23.Chang B Y, Hee W C, Ling R.et al Local anaesthetic techniques and pulsatile ocular blood flow. Br J Ophthalmol 2000841260–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coupland S G, Deschenes M C, Hamilton R C. Impairment of ocular blood flow during regional orbital anesthesia. Can J Ophthalmol 200136140–144. [DOI] [PubMed] [Google Scholar]
- 25.Watkins R, Beigi B, Yates M.et al Intraocular pressure and pulsatile ocular blood flow after retrobulbar and peribulbar anaesthesia. Br J Ophthalmol 200185796–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huber K K, Remky A. Effect of retrobulbar versus subconjunctival anaesthesia on retrobulbar haemodynamics. Br J Ophthalmol 200589719–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Geneva: Department of Noncommunicable Disease Surveillance, WHO, 1999
- 28.Flammer J, Bebie H. Lens opacity meter: a new instrument to quantify lens opacity. Ophthalmologica 198719569–72. [DOI] [PubMed] [Google Scholar]
- 29.Lam A K, Chan S T, Chan H.et al The effect of age on ocular blood supply determined by pulsatile ocular blood flow and color Doppler ultrasonography. Optom Vis Sci 200380305–311. [DOI] [PubMed] [Google Scholar]
- 30.Harris A, Harris M, Biller J.et al Aging affects the retrobulbar circulation differently in women and men. Arch Ophthalmol 20001181076–1080. [DOI] [PubMed] [Google Scholar]
- 31.Matthiessen E T, Zeitz O, Richard G.et al Reproducibility of blood flow velocity measurements using colour decoded Doppler imaging. Eye 200418400–405. [DOI] [PubMed] [Google Scholar]
- 32.Hirsch R P, Schwartz B. Prevalence of circulatory disease among patients undergoing intracapsular cataract extraction. Arch Ophthalmol 19841021015–1018. [DOI] [PubMed] [Google Scholar]
- 33.Street D A, Javitt J C, Wang Q.et al Atherosclerotic disease in patients undergoing cataract extraction. A nationwide case‐control study. The Cataract Patient Outcomes Research Team. Arch Ophthalmol 19961141407–1411. [DOI] [PubMed] [Google Scholar]
- 34.Flammer J, Orgul S, Costa V P.et al The impact of ocular blood flow in glaucoma. Prog Retin Eye Res 200221359–393. [DOI] [PubMed] [Google Scholar]
- 35.Truscott R J. Age‐related nuclear cataract‐oxidation is the key. Exp Eye Res 200580709–725. [DOI] [PubMed] [Google Scholar]
- 36.Varma S D, Hegde K R, Kovtun S. Attenuation and delay of diabetic cataracts by antioxidants: effectiveness of pyruvate after onset of cataract. Ophthalmologica 2005219309–315. [DOI] [PubMed] [Google Scholar]
- 37.Leske M C, Chylack L T, Jr, Wu S Y. The Lens Opacities Case‐Control Study. Risk factors for cataract. Arch Ophthalmol 1991109244–251. [DOI] [PubMed] [Google Scholar]
- 38.Leske M C, Wu S Y, Connell A M.et al Lens opacities, demographic factors and nutritional supplements in the Barbados Eye Study. Int J Epidemiol 1997261314–1322. [DOI] [PubMed] [Google Scholar]
- 39.McCarty C A, Nanjan M B, Taylor H R. Attributable risk estimates for cataract to prioritize medical and public health action. Invest Ophthalmol Vis Sci 2000413720–3725. [PubMed] [Google Scholar]
- 40.The Italian‐American Clinical Trial of Nutritional Supplements and Age‐Related Cataract (CTNS): design implications CTNS report no. 1. Control Clin Trials 200324815–829. [DOI] [PubMed] [Google Scholar]
- 41.Klein B E, Klein R. Cataracts and macular degeneration in older Americans. Arch Ophthalmol 1982100571–573. [DOI] [PubMed] [Google Scholar]
- 42.Ramakrishnan S, Sulochana K N, Selvaraj T.et al Smoking of beedies and cataract: cadmium and vitamin C in the lens and blood. Br J Ophthalmol 199579202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cumming R G, Mitchell P. Alcohol, smoking, and cataracts: the Blue Mountains Eye Study. Arch Ophthalmol 19971151296–1303. [DOI] [PubMed] [Google Scholar]
- 44.West S K, Valmadrid C T. Epidemiology of risk factors for age‐related cataract. Surv Ophthalmol 199539323–334. [DOI] [PubMed] [Google Scholar]
- 45.Hiller R, Sperduto R D, Podgor M J.et al Cigarette smoking and the risk of development of lens opacities. The Framingham studies. Arch Ophthalmol 19971151113–1118. [DOI] [PubMed] [Google Scholar]