Abstract
Objective
To measure the human lamina cribrosa thickness (LCT) in vitro in fully hydrated specimens and to determine whether there is any association between thickness and age or sex.
Methods
45 fixed human optic nerves, age range 9–90 years, were dissected from the globe and frozen sectioned. The study was divided into two parts: the first investigated the overall change in LCT and cribrosal beam thickness (CBT) with age, and the second divided eyes into two specific age groups (38–49 and 78–87 years) and assessed differences with respect to age and sex.
Results
LCT ranged from 345.4 to 555.9 μm between the samples. A positive relationship was found between LCT and age (LCT = 2.41×age+365.5, 95% confidence interval (CI) for slope 1.31 to 3.52; r2 = 0.30, p<0.001). A regional difference in CBT was observed, with beams being thickest at the posterior cribrosa (mean 14.8 (standard deviation (SD) 2.2) μm) and thinnest at the anterior cribrosa (9.8 (SD 2.4) μm). CBT increased with increasing age. Differences related to sex were also found, with females having relatively thinner LCT than males, irrespective of age, but this was not statistically significant.
Conclusions
This study shows an increase in human LCT with increasing age. This changing structural property of the lamina cribrosa may have implications for its functioning with respect to compliance and reversibility, and has particular relevance to glaucoma, where increasing age has been identified as a strong risk factor for the development of the disease.
The lamina cribrosa is a series of perforated collagenous plates through which the retinal blood vessels and ganglion cell axons exit the eye.1,2,3 In glaucoma, it has been implicated as one of the main sites of damage to the ganglion cell axons, particularly in patients with high‐tension glaucoma where it is thought that the raised intraocular pressure results in a deformation of the structure and shearing of individual plates in relation to one another.4,5,6 However, the exact mechanism of how this deformation results in axonal death is still unclear.
Studies have shown that with increasing age, there is an increase in collagen type I in the cribrosal plates7,8 and an increase in the total collagen content of the lamina cribrosa.9 However, there is no comprehensive description of how the overall configuration of this structure changes with age. This study investigated whether any architectural differences in the lamina cribrosa occurred with increasing age.
Materials and methods
Forty five human cadaver eyes were received from the corneal transplant service eye bank at the Moorfields Eye Hospital, London, UK, and Bristol Eye Hospitals, Bristol, UK, after corneas had been removed for transplantation. In cases where pairs of eyes had been received, only one eye from the pair was used in the study. Eyes were collected within approximately 48 h of death and stored in 10% formaldehyde in phosphate‐buffered saline (PBS; pH 7.2). Full local ethical committee consent was obtained for research use. The samples had no history of ocular abnormality, and optic nerves and retinas appeared normal by gross examination during dissection.
The study was divided into two parts: (1) an exploratory study to examine whether there were any changes in the central thickness of the lamina cribrosa and cribrosal beams with increasing age and (2) a second study to examine these features of the lamina cribrosa in two specific age and sex groups.
Tissue preparation
Optic nerve heads were dissected from the globe and stored in a solution of 20% sucrose in PBS overnight for cryoprotection.10 Nerves were then embedded in gel–albumin/2.5% glutaraldehyde to provide a supporting medium for frozen sectioning. Sections parallel to the long axis of the optic nerve, 50 μm thick, were frozen microtomed. Every fifth section was collected and stored in 10% formaldehyde in individual numbered wells. Wet sections were mounted on slides using PBS and a standard cover glass, and examined in a fully hydrated condition by light microscopy.
Study I: thickness of lamina cribrosa with age
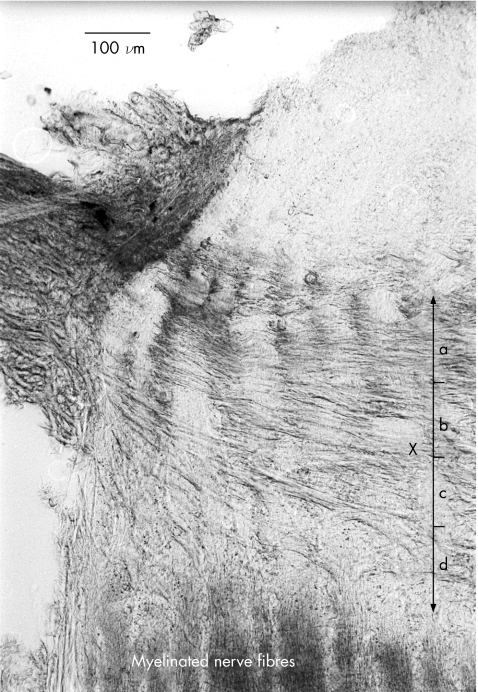
Twenty seven eyes were used for this study. The change in collagen architecture around the central retinal artery and vein make demarcation of the laminal difficult, so sections involving this area were avoided. In all, 4–8 microtomed sections, 100 μm on either side of the exit of the central retinal artery, were measured for each eye, and measurements were started 100 μm in from the scleral border of the optic nerve, resulting in about 100 measurements per eye. Lamina cribrosa thickness (LCT), defined as the measurement from the first collagenous plate to the point where myelinated axons became visible (fig 1), was measured in 50‐μm steps across each section using a graticule fixed in the eyepiece of the microscope. The average of these measurements and the 95% confidence intervals (CIs) were plotted against age.
Figure 1 Section of lamina cribrosa illustrating lamina cribrosa thickness (LCT) measurement. LCT was defined as the measurement from the first collagenous plate to the point where myelinated axons became visible, delineated by “X”. The area was arbitrarily divided into four sections (a–d), about 100 μm thick, for measurement of cribrosal beam thickness. Magnification 10×. a, anterior; b, mid‐anterior; c, mid‐posterior; d, posterior.
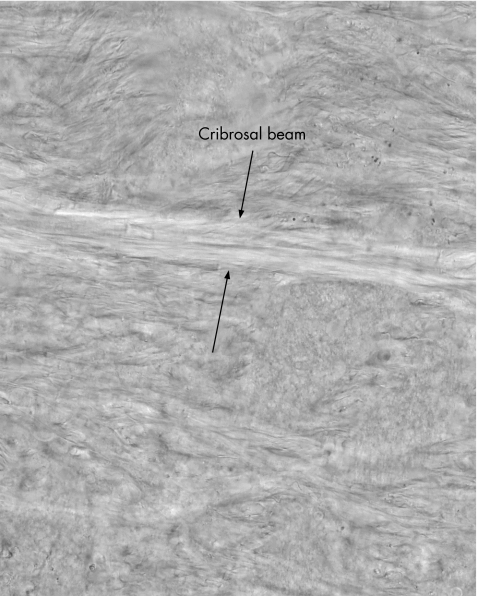
In these preparations, it was possible to distinguish discrete layers within the lamina cribrosa. A second measurement was taken in 12 of the 27 eyes of the thickness of these discrete areas, which we termed “cribrosal beams”. One tissue sample from each eye was mounted on a wet slide and examined under a light microscope connected to a computer via a charged‐coupled device attachment at 40× magnification. The lamina cribrosa area was divided into four areas: anterior, mid‐anterior, mid‐posterior and posterior. All regions were about 100 μm thick (figs 1, 2). Single images in each region were grabbed and cribrosal beam thickness (CBT) was determined using Matrox Inspector Imaging Software V.2.1 (Matrox Electronic Systems, Quebec, Canada). The observer (AK) was masked to the age of the samples.
Figure 2 The cribrosal beam measurements. Cribrosal beams could be clearly identified in sections under differential interference contrast illumination. Beam thickness was measured perpendicular to edges of beam in four arbitrary sections (as in fig 1). Magnification ×40.
Study II: comparison of LCT in two age‐specific and sex‐specific groups
In a separate study 18 eyes were used, selected from both sexes in age categories of 38–49 and 78–89 years. The age limits used to segregate eyes into “relatively young” and “relatively old” groups were determined by tissue availability. Two tissue sections from each eye, 150 μm on either side of the central retinal artery section, were examined. The observer (SI) was masked to the age and sex of the samples.
Data analysis
Data were statistically analysed using MedCalc V.8.0.0.1 (MedCalc, Mariakerke, Belgium). Linear regression analysis was used to determine the relationship between LCT and age. The data showed a normal distribution (Kolmogorov–Smirnov test from the statistical package), therefore a paired t test was used for the analysis of regional measurements of CBT and an unpaired t test was used for the analysis of differences in measurements of age‐specific and sex‐specific groups.
Results
Study I: thickness of lamina cribrosa with age
In this sample of 27 eyes, with an age range of 9–90 years, LCT ranged between 345.4 and 555.9 μm, with a mean (standard deviation (SD)) of 451.3 (56.5) μm (table 1).
Table 1 Age range and section details for study I (n = 27).
| Mean | SD | Range | |
|---|---|---|---|
| Age (years) | 53.7 | 25.2 | 9–90 |
| Diameter of specimen sections (mm) | 1.55 | 0.23 | 1.20–1.95 |
| Number of LCT measurements per eye | 111 | 33 | 80–176 |
| LCT (μm) | 451.3 | 56.5 | 345.4–555.9 |
LCT, lamina cribrosa thickness.
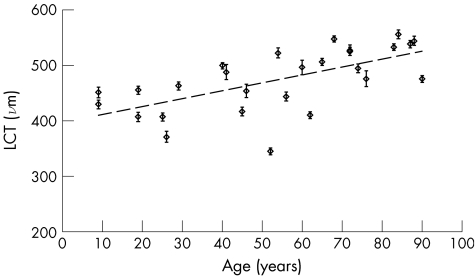
LCT increased with increasing age (fig 3). The relationship between LCT and age indicates a 14‐μm increase per decade (LCT = 1.42×age+397.1, 95% CI for slope 0.75 to 2.10; r2 = 0.40, p<0.001).
Figure 3 Change in lamina cribrosa thickness (LCT) with age. Results from study I. Error bars show 95% confidence limits of measurements in each eye. Regression line: LCT = 1.42×age +397.1, 95% CI for slope 0.75 to 2.10; r2 = 0.40, p<0.001.
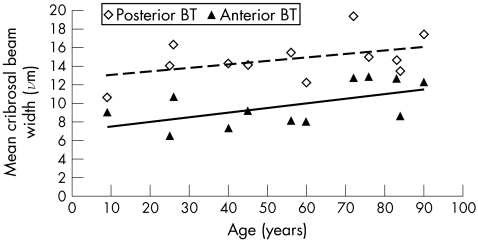
We found a regional difference in beam thickness, with beams being thickest at the posterior cribrosa (range 12.4–19.4, mean 14.8, SD 2.2 μm) and thinnest at the anterior cribrosa (range 6.4–12.8, mean 9.8, SD 2.4 μm). The difference between the CBT in the posterior and anterior sections of the lamina was significant (mean difference 5 μm, paired t test p<0.001). There was a trend showing an increase in CBT with age in all four regions (anterior, mid‐anterior, mid‐posterior and posterior). CBT increased on average 0.5 μm per decade in each region. For simplicity, fig 4 shows the change in only the anterior and posterior beam thicknesses.
Figure 4 Mean cribrosal beam thickness with age. Data shown are cribrosal beam thickness values in posterior and anterior regions of the lamina cribrosa (regions defined in fig 1) from a subset of eyes from study I (n = 12). BT, beam thickness. Linear regression analysis: anterior BT = 0.05×age+7.0; r2 = 0.33, p = 0.05; mid‐anterior BT = 0.047×age+7.9; r2 = 0.27, p = 0.09; mid‐posterior BT = 0.064×age +8.9; r2 = 0.43, p = 0.02; posterior BT = 0.039×age+12.6; r2 = 0.20, p = 0.14.
Study II: comparison of LCT in two age‐specific and sex‐specific groups
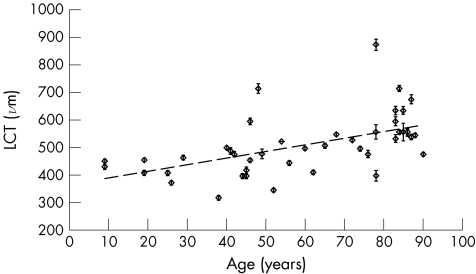
We found a significant difference in mean LCT between the two age groups, with older eyes having a significantly thicker LCT than younger eyes, consistent with the findings from study I (group 1, age 38–49 years, mean 481.7 (SD 134.4) μm; group 2, age 78–87 years, mean 613.5 (SD 119.4) μm; unpaired t test p<0.05; fig 5). Pooling the data from both studies strengthened the finding that LCT increases with increasing age, although in this second regression there are more samples from the two older age groups (fig 6; LCT = 2.41×age+365.5, 95% CI for slope 1.31 to 3.52; r2 = 0.30, p<0.001).
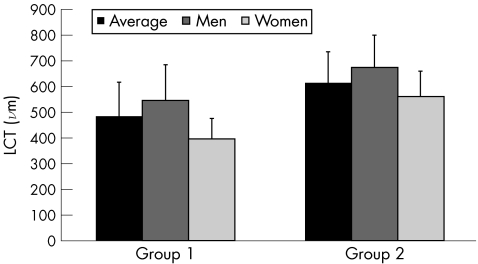
Figure 5 Differences with respect to sex in lamina cribrosa thickness (LCT) in each group. Group 1: 38–49 years, four men and three women. Group 2: 78–87 years, five men and six women.
Figure 6 Change in lamina cribrosa thickness (LCT) with age—pooled data. Results of studies I and II are pooled together. There are more samples in the 38–49‐year and 78–89‐year age groups. Error bars show 95% CI of measurements in each eye. Regression line: LCT = 2.41×age+365.5, 95% CI for slope 1.31 to 3.52; r2 = 0.30, p<0.001.
In both age groups, eyes of men had greater LCT than those of women, although this did not reach statistical significance (group 1: mean male LCT 545.1 (SD 138.7) μm v female LCT 396.7 (SD 79.5) μm; group 2: mean male LCT 674.6 (SD 125.4) μm v 562.5 (95.3) μm; fig 5).
Discussion
This is the first study on fully hydrated human lamina cribrosa tissue that shows an increase in the actual thickness of the human lamina cribrosa with increasing age. In addition, differences regarding sex were also observed, with males having a thicker lamina cribrosa than females, although this finding did not reach statistical significance.
Wilczek1 analysed the human lamina cribrosa in detail in 1947 describing the organisation of lamina pores by generating a three‐dimensional beeswax model of this structure from dehydrated histological sections. These studies have been extended by others who have carried out detailed analyses using both scanning and transmission electron microscopy.4,5,6,11 Although these have provided valuable data of both quantitative and qualitative nature, each study was undertaken on dehydrated tissue. In some cases tissue was processed for wax embedding or electron microscopy, both of which can induce tissue distortion resulting from digestion and dehydration of tissue during its preparation. Shrinkage from dehydration alone can reduce the extracellular matrix by up to 30%.12 Our finding of thicknesses about 50% greater than this estimate may arise as a result of our use of hydrated material.
Our definition of the measured limits of LCT is based on evidence that the lamina cribrosa acts as a barrier to oligodendroglia migration into the eye, thus serving as a boundary between the unmyelinated ganglion cell axon and the myelinated portion of the axon in the optic nerve and tract.13 To our knowledge, no evidence yet suggests that myelination of ganglion cell axons recede during the normal ageing process of the human eye. Although it is impossible to control for the effect of postmortem changes that take place before fixation, these may be relatively minor, and the results of this study may provide a good estimate for the thickness of the lamina cribrosa as found in situ. There are no reasons to believe that the age‐related changes reported here are due to artefact.
Our finding of an increase in LCT with increasing age correlates well with results of immunohistochemical and biochemical studies on human lamina cribrosa tissue, which have shown that collagen in the lamina cribrosa accumulates after birth and increases throughout life.7,8,9,14 It has been observed that the appearance of the lamina cribrosa differs through its thickness and that the lamina cribrosa beams are less dense in the anterior lamina than in the posterior lamina.6 Our results confirm this difference in beam thickness through the lamina cribrosa and also that beam thickness increased with increasing age in a subset of eyes. Several studies on human and primate optic nerves have found a thickening of the trabeculae of the fibrous connective tissue fascicles of the optic nerve with increasing age,15,16,17 so it would be reasonable to find a similar increase in CBT with increasing age.
Burgoyne et al18,19 described a model of the optic nerve as a biomechanical structure and showed how stress and strain forces generated by intraocular pressure are concentrated around the peripapillary sclera, the scleral canal and the lamina cribrosa, often described as the “load‐bearing” connective tissues. The material properties of these load‐bearing tissues will determine their mechanical response to different levels of intraocular pressure. An increase in CBT with increasing age may result in a change in lamina cribrosa behaviour. An age‐related decline in the compliance of the lamina cribrosa, assessed using confocal scanning ophthalmoscopy, has been documented previously.20 In certain susceptible eyes, this increase in the age‐related thickness in the lamina beams may result in an exaggerated response to changes in intraocular pressure, leading to permanent deformation of the lamina cribrosa, and ultimately resulting in axonal loss.
This change in the structural property of the ageing lamina cribrosa may offer a partial explanation for the age‐related decline of nerve fibres.21 The optic nerve axons obtain their nutrients from the lamina capillaries22 and an increase in LCT may reduce the effectiveness of diffusion of these nutrients from the capillaries resulting in a gradual decay of nerve fibres. This effect may be exaggerated in patients with vasospastic conditions leading to the “senile sclerotic” glaucomatous optic disc appearance, characterised by a shallow, saucerised cupping and pallor, rather than an excavation of the disc.23
Our study also showed a greater LCT in males than in females, irrespective of age. Sex‐specific differences in ocular parameters have been reported previously, with males showing slightly larger optic discs.24 Our results of sex‐specific differences may be more a reflection of differences in the physical characteristics of the optic nerve head between males and females, and should be the subject of further investigation.
In summary, this cross‐sectional study of the architectural changes in the human lamina cribrosa suggests that there is an increase in both the cribrosal beam and overall LCT with increasing age. These changes may have implications for functioning of the lamina cribrosa with respect to compliance and reversibility and should be further studied. Further studies are required to establish the regional differences in thickness.
Acknowledgements
We are grateful for the assistance from the Moorfields Eye Bank and Bristol Eye Bank.
Abbreviations
CBT - cribrosal beam thickness
LCT - lamina cribrosa thickness
PBS - phosphate‐buffered saline
Footnotes
Competing interests: None declared.
References
- 1.Wilczek M. The lamina cribrosa and its nature. Br J Ophthalmol 194731551–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson D R. Ultrastructure of human and monkey lamina cribrosa and optic nerve head. Arch Ophthalmol 196982800–814. [DOI] [PubMed] [Google Scholar]
- 3.Emery J M, Landis D, Paton D.et al The lamina cribrosa in normal and glaucomatous human eyes. Trans Am Acad Ophthalmol Otolaryngol 197478Op290–Op297. [PubMed] [Google Scholar]
- 4.Quigley H A, Addicks E M, Green W R.et al Optic nerve damage in human glaucoma. II. The site of injury and susceptibility to damage. Arch Ophthalmol 198199635–649. [DOI] [PubMed] [Google Scholar]
- 5.Quigley H A, Hohman R M, Addicks E M.et al Morphologic changes in the lamina cribrosa correlated with neural loss in open‐angle glaucoma. Am J Ophthalmol 198395673–691. [DOI] [PubMed] [Google Scholar]
- 6.Radius R L, Gonzales M. Anatomy of the lamina cribrosa in human eyes. Arch Ophthalmol 1981992159–2162. [DOI] [PubMed] [Google Scholar]
- 7.Hernandez M R, Luo X X, Andrzejewska W.et al Age‐related changes in the extracellular matrix of the human optic nerve head. Am J Ophthalmol 1989107476–484. [DOI] [PubMed] [Google Scholar]
- 8.Morrison J C, Jerdan J A, Dorman M E.et al Structural proteins of the neonatal and adult lamina cribrosa. Arch Ophthalmol 19891071220–1224. [DOI] [PubMed] [Google Scholar]
- 9.Albon J, Karwatowski W S, Avery N.et al Changes in the collagenous matrix of the aging human lamina cribrosa. Br J Ophthalmol 199579368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bancroft J D, Stevens A. eds. Theory and practice of histology techniques. 4th edn. London: Churchill Livingstone, 1996
- 11.Quigley H A, Addicks E M. Regional differences in the structure of the lamina cribrosa and their relation to glaucomatous optic nerve damage. Arch Ophthalmol 198199137–143. [DOI] [PubMed] [Google Scholar]
- 12.Birch M, Brotchie D, Roberts N.et al The three‐dimensional structure of the connective tissue in the lamina cribrosa of the human optic nerve head. Ophthalmologica 1997211183–191. [DOI] [PubMed] [Google Scholar]
- 13.Perry V H, Lund R D. Evidence that the lamina cribrosa prevents intraretinal myelination of retinal ganglion cell axons. J Neurocytol 199019265–272. [DOI] [PubMed] [Google Scholar]
- 14.Ogden T E, Duggan J, Danley K.et al Morphometry of nerve fiber bundle pores in the optic nerve head of the human. Exp Eye Res 198846559–568. [DOI] [PubMed] [Google Scholar]
- 15.Dolman C L, McCormick A Q, Drance S M. Aging of the optic nerve. Arch Ophthalmol 1980982053–2058. [DOI] [PubMed] [Google Scholar]
- 16.Munari P F, De Caro R, Colletti D. Anatomy of the optic nerve in elderly men. Metab Pediatr Syst Ophthalmol 19891213–16. [PubMed] [Google Scholar]
- 17.Sandell J H, Peters A. Effects of age on nerve fibers in the rhesus monkey optic nerve. J Comp Neurol 2001429541–553. [DOI] [PubMed] [Google Scholar]
- 18.Burgoyne C F, Downs J C, Bellezza A J.et al The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP‐related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retin Eye Res 20052439–73. [DOI] [PubMed] [Google Scholar]
- 19.Bellezza A J, Hart R T, Burgoyne C F. The optic nerve head as a biomechanical structure: initial finite element modeling. Invest Ophthalmol Vis Sci 2000412991–3000. [PubMed] [Google Scholar]
- 20.Albon J, Purslow P P, Karwatowski W S.et al Age related compliance of the lamina cribrosa in human eyes. Br J Ophthalmol 200084318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garway‐Heath D F, Wollstein G, Hitchings R A. Aging changes of the optic nerve head in relation to open angle glaucoma. Br J Ophthalmol 199781840–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cioffi G A, Van Buskirk E M. Vasculature of the anterios optic nerve and peripapillary choroid. In: Ritch R, Shields MB, Krupin T, eds. The glaucomas. Mosby: St Loius, 1996177–188.
- 23.Broadway D C, Drance S M. Glaucoma and vasospasm. Br J Ophthalmol 199882862–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varma R, Tielsch J M, Quigley H A.et al Race‐, age‐, gender‐, and refractive error‐related differences in the normal optic disc. Arch Ophthalmol 19941121068–1076. [DOI] [PubMed] [Google Scholar]