Abstract
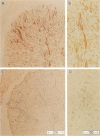
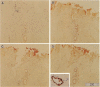
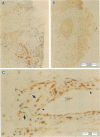
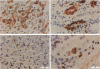
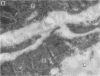
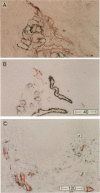
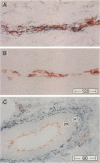
Some human chronic dermal wounds treated with recombinant platelet-derived growth factor-BB (rPDGF-BB) show increased healing coupled with fibroblast activation and granulation tissue formation. To determine whether endogenous PDGF is associated with healing and nonhealing dermal ulcer phenotypes, we developed monoclonal antibodies capable of recognizing the three isoforms of PDGF, AA, AB, and BB dimers, and capable of discriminating between two alternatively spliced A chain transcripts. We detected little PDGF isoform expression in normal skin and in nonhealing dermal ulcers. In contrast, in surgically created acute wounds and chronic ulcers treated with rPDGF-BB, markedly upregulated levels of PDGF-AA (long form) were found. In both types of wounds, increased PDGF-AA was detected primarily in capillaries and fibroblasts, although in rPDGF-BB-treated chronic wounds, widespread expression of PDGF-AA was somewhat delayed. With continued treatment, the long form of PDGF-AA, which can preferentially bind extracellular matrix, was expressed only in capillaries, while fibroblasts began synthesizing the short form of PDGF-AA. Within capillaries, all endothelial cells and varying numbers of pericytes and smooth muscle cells contained PDGF-AA. In all wounds, macrophages and keratinocytes were not a major contributor. While PDGF-BB and PDGF-AB were present in a minority of healing wounds, they were usually present at lower levels than PDGF-AA. PDGF-beta receptors, which bind only PDGF-BB and not other isoforms, were found in normal skin and granulation tissue, providing a molecular basis for treating human chronic wounds with exogenous rPDGF-BB.
Full text
PDF

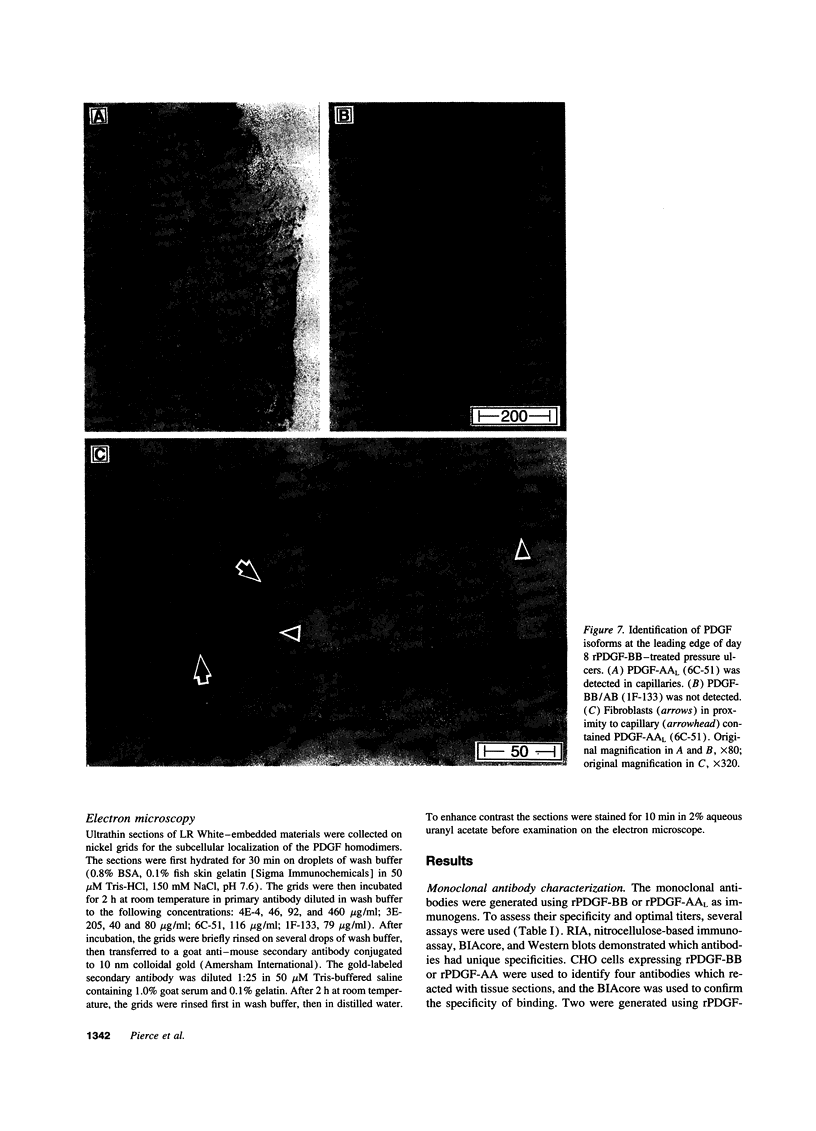
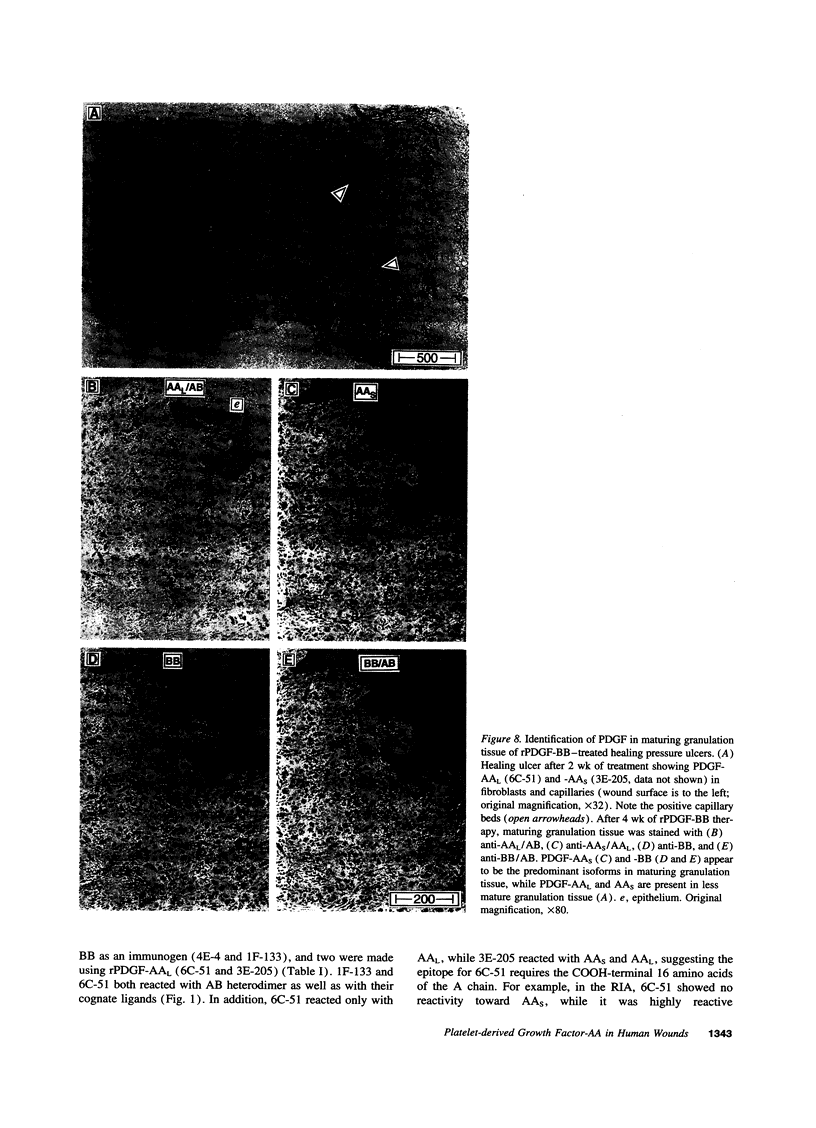

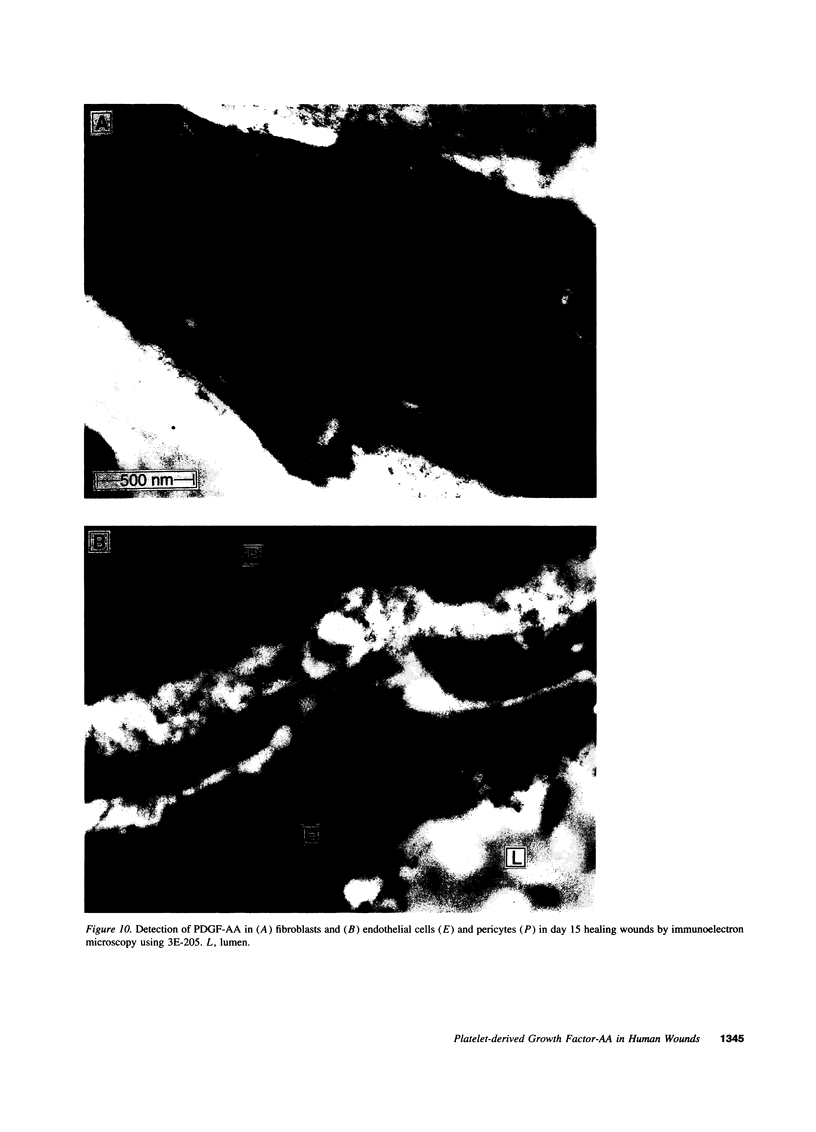


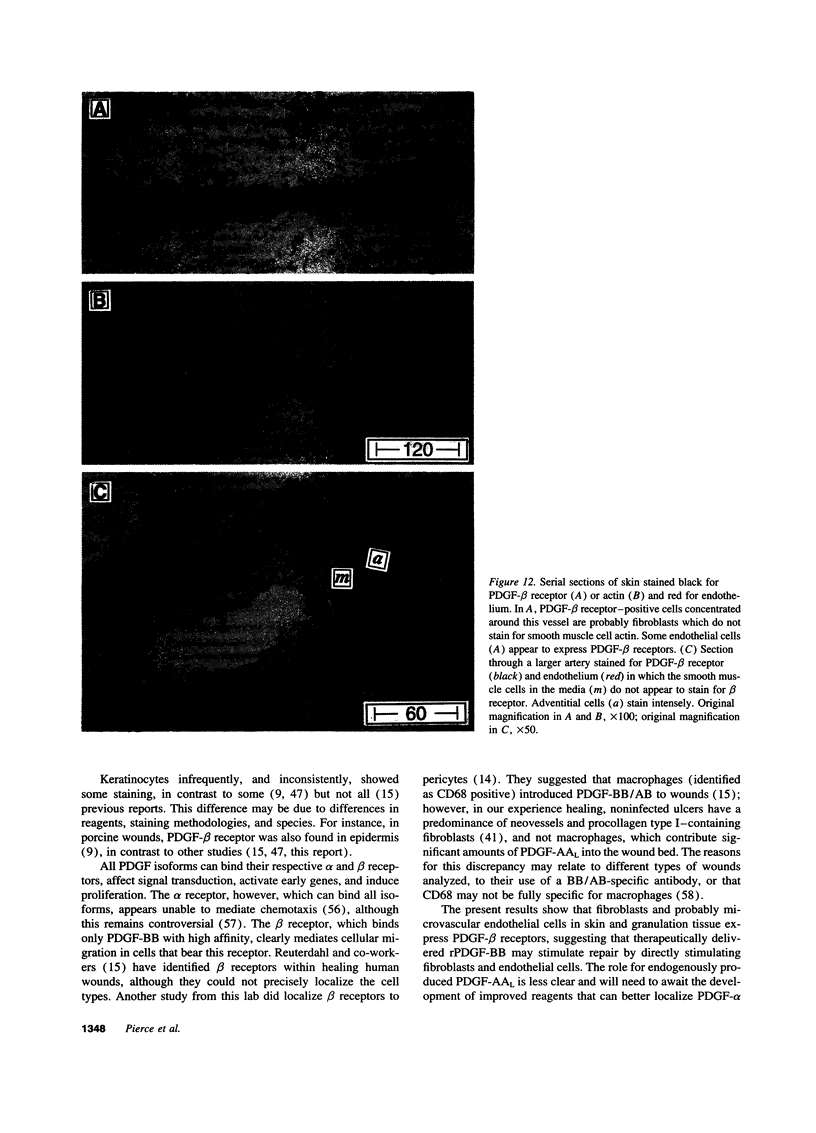


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansel J. C., Tiesman J. P., Olerud J. E., Krueger J. G., Krane J. F., Tara D. C., Shipley G. D., Gilbertson D., Usui M. L., Hart C. E. Human keratinocytes are a major source of cutaneous platelet-derived growth factor. J Clin Invest. 1993 Aug;92(2):671–678. doi: 10.1172/JCI116636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniades H. N., Galanopoulos T., Neville-Golden J., Kiritsy C. P., Lynch S. E. Injury induces in vivo expression of platelet-derived growth factor (PDGF) and PDGF receptor mRNAs in skin epithelial cells and PDGF mRNA in connective tissue fibroblasts. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):565–569. doi: 10.1073/pnas.88.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigham-Burke M., Edwards J. R., O'Shannessy D. J. Detection of receptor-ligand interactions using surface plasmon resonance: model studies employing the HIV-1 gp120/CD4 interaction. Anal Biochem. 1992 Aug 15;205(1):125–131. doi: 10.1016/0003-2697(92)90588-x. [DOI] [PubMed] [Google Scholar]
- Broadley K. N., Aquino A. M., Woodward S. C., Buckley-Sturrock A., Sato Y., Rifkin D. B., Davidson J. M. Monospecific antibodies implicate basic fibroblast growth factor in normal wound repair. Lab Invest. 1989 Nov;61(5):571–575. [PubMed] [Google Scholar]
- Bywater M., Rorsman F., Bongcam-Rudloff E., Mark G., Hammacher A., Heldin C. H., Westermark B., Betsholtz C. Expression of recombinant platelet-derived growth factor A- and B-chain homodimers in rat-1 cells and human fibroblasts reveals differences in protein processing and autocrine effects. Mol Cell Biol. 1988 Jul;8(7):2753–2762. doi: 10.1128/mcb.8.7.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A. Basics of cutaneous wound repair. J Dermatol Surg Oncol. 1993 Aug;19(8):693–706. doi: 10.1111/j.1524-4725.1993.tb00413.x. [DOI] [PubMed] [Google Scholar]
- Collins T., Bonthron D. T., Orkin S. H. Alternative RNA splicing affects function of encoded platelet-derived growth factor A chain. Nature. 1987 Aug 13;328(6131):621–624. doi: 10.1038/328621a0. [DOI] [PubMed] [Google Scholar]
- Cooper D. M., Yu E. Z., Hennessey P., Ko F., Robson M. C. Determination of endogenous cytokines in chronic wounds. Ann Surg. 1994 Jun;219(6):688–692. doi: 10.1097/00000658-199406000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amore P. A., Smith S. R. Growth factor effects on cells of the vascular wall: a survey. Growth Factors. 1993;8(1):61–75. doi: 10.3109/08977199309029135. [DOI] [PubMed] [Google Scholar]
- Deuel T. F., Kawahara R. S., Mustoe T. A., Pierce A. F. Growth factors and wound healing: platelet-derived growth factor as a model cytokine. Annu Rev Med. 1991;42:567–584. doi: 10.1146/annurev.me.42.020191.003031. [DOI] [PubMed] [Google Scholar]
- Eriksson A., Siegbahn A., Westermark B., Heldin C. H., Claesson-Welsh L. PDGF alpha- and beta-receptors activate unique and common signal transduction pathways. EMBO J. 1992 Feb;11(2):543–550. doi: 10.1002/j.1460-2075.1992.tb05085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg K., Valyi-Nagy I., Heldin C. H., Herlyn M., Westermark B. Platelet-derived growth factor (PDGF) in oncogenesis: development of a vascular connective tissue stroma in xenotransplanted human melanoma producing PDGF-BB. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):393–397. doi: 10.1073/pnas.90.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden M. A., Au Y. P., Kirkman T. R., Wilcox J. N., Raines E. W., Ross R., Clowes A. W. Platelet-derived growth factor activity and mRNA expression in healing vascular grafts in baboons. Association in vivo of platelet-derived growth factor mRNA and protein with cellular proliferation. J Clin Invest. 1991 Feb;87(2):406–414. doi: 10.1172/JCI115011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhalgh D. G., Sprugel K. H., Murray M. J., Ross R. PDGF and FGF stimulate wound healing in the genetically diabetic mouse. Am J Pathol. 1990 Jun;136(6):1235–1246. [PMC free article] [PubMed] [Google Scholar]
- Grotendorst G. R., Martin G. R., Pencev D., Sodek J., Harvey A. K. Stimulation of granulation tissue formation by platelet-derived growth factor in normal and diabetic rats. J Clin Invest. 1985 Dec;76(6):2323–2329. doi: 10.1172/JCI112243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart C. E., Bailey M., Curtis D. A., Osborn S., Raines E., Ross R., Forstrom J. W. Purification of PDGF-AB and PDGF-BB from human platelet extracts and identification of all three PDGF dimers in human platelets. Biochemistry. 1990 Jan 9;29(1):166–172. doi: 10.1021/bi00453a022. [DOI] [PubMed] [Google Scholar]
- Hart C. E., Forstrom J. W., Kelly J. D., Seifert R. A., Smith R. A., Ross R., Murray M. J., Bowen-Pope D. F. Two classes of PDGF receptor recognize different isoforms of PDGF. Science. 1988 Jun 10;240(4858):1529–1531. doi: 10.1126/science.2836952. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Bäckström G., Ostman A., Hammacher A., Rönnstrand L., Rubin K., Nistér M., Westermark B. Binding of different dimeric forms of PDGF to human fibroblasts: evidence for two separate receptor types. EMBO J. 1988 May;7(5):1387–1393. doi: 10.1002/j.1460-2075.1988.tb02955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermiston M. L., Latham C. B., Gordon J. I., Roth K. A. Simultaneous localization of six antigens in single sections of transgenic mouse intestine using a combination of light and fluorescence microscopy. J Histochem Cytochem. 1992 Sep;40(9):1283–1290. doi: 10.1177/40.9.1506665. [DOI] [PubMed] [Google Scholar]
- Kelly J. L., Sánchez A., Brown G. S., Chesterman C. N., Sleigh M. J. Accumulation of PDGF B and cell-binding forms of PDGF A in the extracellular matrix. J Cell Biol. 1993 Jun;121(5):1153–1163. doi: 10.1083/jcb.121.5.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khachigian L. M., Chesterman C. N. Platelet-derived growth factor and alternative splicing: a review. Pathology. 1992 Oct;24(4):280–290. doi: 10.3109/00313029209068882. [DOI] [PubMed] [Google Scholar]
- Kohler N., Lipton A. Platelets as a source of fibroblast growth-promoting activity. Exp Cell Res. 1974 Aug;87(2):297–301. doi: 10.1016/0014-4827(74)90484-4. [DOI] [PubMed] [Google Scholar]
- Leibovich S. J., Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol. 1975 Jan;78(1):71–100. [PMC free article] [PubMed] [Google Scholar]
- Matoskova B., Rorsman F., Svensson V., Betsholtz C. Alternative splicing of the platelet-derived growth factor A-chain transcript occurs in normal as well as tumor cells and is conserved among mammalian species. Mol Cell Biol. 1989 Jul;9(7):3148–3150. doi: 10.1128/mcb.9.7.3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T., Pierce J. H., Fleming T. P., Greenberger J. S., LaRochelle W. J., Ruggiero M., Aaronson S. A. Independent expression of human alpha or beta platelet-derived growth factor receptor cDNAs in a naive hematopoietic cell leads to functional coupling with mitogenic and chemotactic signaling pathways. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8314–8318. doi: 10.1073/pnas.86.21.8314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery E. A., Meis J. M., Mitchell M. S., Enzinger F. M. Atypical decubital fibroplasia. A distinctive fibroblastic pseudotumor occurring in debilitated patients. Am J Surg Pathol. 1992 Jul;16(7):708–715. [PubMed] [Google Scholar]
- Mustoe T. A., Cutler N. R., Allman R. M., Goode P. S., Deuel T. F., Prause J. A., Bear M., Serdar C. M., Pierce G. F. A phase II study to evaluate recombinant platelet-derived growth factor-BB in the treatment of stage 3 and 4 pressure ulcers. Arch Surg. 1994 Feb;129(2):213–219. doi: 10.1001/archsurg.1994.01420260109015. [DOI] [PubMed] [Google Scholar]
- Mustoe T. A., Pierce G. F., Morishima C., Deuel T. F. Growth factor-induced acceleration of tissue repair through direct and inductive activities in a rabbit dermal ulcer model. J Clin Invest. 1991 Feb;87(2):694–703. doi: 10.1172/JCI115048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaoka I., Someya A., Iwabuchi K., Yamashita T. Comparative studies on the platelet-derived growth factor-A and -B gene expression in human monocytes. Comp Biochem Physiol B. 1991;100(2):313–319. doi: 10.1016/0305-0491(91)90381-m. [DOI] [PubMed] [Google Scholar]
- Nakahara K., Nishimura H., Kuro-o M., Takewaki S., Iwase M., Ohkubo A., Yazaki Y., Nagai R. Identification of three types of PDGF-A chain gene transcripts in rabbit vascular smooth muscle and their regulated expression during development and by angiotensin II. Biochem Biophys Res Commun. 1992 Apr 30;184(2):811–818. doi: 10.1016/0006-291x(92)90662-5. [DOI] [PubMed] [Google Scholar]
- Nistér M., Hammacher A., Mellström K., Siegbahn A., Rönnstrand L., Westermark B., Heldin C. H. A glioma-derived PDGF A chain homodimer has different functional activities from a PDGF AB heterodimer purified from human platelets. Cell. 1988 Mar 25;52(6):791–799. doi: 10.1016/0092-8674(88)90421-7. [DOI] [PubMed] [Google Scholar]
- Ostman A., Bäckström G., Fong N., Betsholtz C., Wernstedt C., Hellman U., Westermark B., Valenzuela P., Heldin C. H. Expression of three recombinant homodimeric isoforms of PDGF in Saccharomyces cerevisiae: evidence for difference in receptor binding and functional activities. Growth Factors. 1989;1(3):271–281. doi: 10.3109/08977198908998003. [DOI] [PubMed] [Google Scholar]
- Paulsson Y., Hammacher A., Heldin C. H., Westermark B. Possible positive autocrine feedback in the prereplicative phase of human fibroblasts. Nature. 1987 Aug 20;328(6132):715–717. doi: 10.1038/328715a0. [DOI] [PubMed] [Google Scholar]
- Pierce G. F., Mustoe T. A., Lingelbach J., Masakowski V. R., Griffin G. L., Senior R. M., Deuel T. F. Platelet-derived growth factor and transforming growth factor-beta enhance tissue repair activities by unique mechanisms. J Cell Biol. 1989 Jul;109(1):429–440. doi: 10.1083/jcb.109.1.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce G. F., Mustoe T. A. Pharmacologic enhancement of wound healing. Annu Rev Med. 1995;46:467–481. doi: 10.1146/annurev.med.46.1.467. [DOI] [PubMed] [Google Scholar]
- Pierce G. F., Mustoe T. A., Senior R. M., Reed J., Griffin G. L., Thomason A., Deuel T. F. In vivo incisional wound healing augmented by platelet-derived growth factor and recombinant c-sis gene homodimeric proteins. J Exp Med. 1988 Mar 1;167(3):974–987. doi: 10.1084/jem.167.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce G. F., Tarpley J. E., Allman R. M., Goode P. S., Serdar C. M., Morris B., Mustoe T. A., Vande Berg J. Tissue repair processes in healing chronic pressure ulcers treated with recombinant platelet-derived growth factor BB. Am J Pathol. 1994 Dec;145(6):1399–1410. [PMC free article] [PubMed] [Google Scholar]
- Pierce G. F., Tarpley J. E., Yanagihara D., Mustoe T. A., Fox G. M., Thomason A. Platelet-derived growth factor (BB homodimer), transforming growth factor-beta 1, and basic fibroblast growth factor in dermal wound healing. Neovessel and matrix formation and cessation of repair. Am J Pathol. 1992 Jun;140(6):1375–1388. [PMC free article] [PubMed] [Google Scholar]
- Pierce G. F., Vande Berg J., Rudolph R., Tarpley J., Mustoe T. A. Platelet-derived growth factor-BB and transforming growth factor beta 1 selectively modulate glycosaminoglycans, collagen, and myofibroblasts in excisional wounds. Am J Pathol. 1991 Mar;138(3):629–646. [PMC free article] [PubMed] [Google Scholar]
- Quaglino D., Jr, Nanney L. B., Kennedy R., Davidson J. M. Transforming growth factor-beta stimulates wound healing and modulates extracellular matrix gene expression in pig skin. I. Excisional wound model. Lab Invest. 1990 Sep;63(3):307–319. [PubMed] [Google Scholar]
- Raines E. W., Ross R. Compartmentalization of PDGF on extracellular binding sites dependent on exon-6-encoded sequences. J Cell Biol. 1992 Jan;116(2):533–543. doi: 10.1083/jcb.116.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuterdahl C., Sundberg C., Rubin K., Funa K., Gerdin B. Tissue localization of beta receptors for platelet-derived growth factor and platelet-derived growth factor B chain during wound repair in humans. J Clin Invest. 1993 May;91(5):2065–2075. doi: 10.1172/JCI116429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson M. C., Phillips L. G., Thomason A., Robson L. E., Pierce G. F. Platelet-derived growth factor BB for the treatment of chronic pressure ulcers. Lancet. 1992 Jan 4;339(8784):23–25. doi: 10.1016/0140-6736(92)90143-q. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J., Kariya B., Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1207–1210. doi: 10.1073/pnas.71.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Masuda J., Raines E. W., Gown A. M., Katsuda S., Sasahara M., Malden L. T., Masuko H., Sato H. Localization of PDGF-B protein in macrophages in all phases of atherogenesis. Science. 1990 May 25;248(4958):1009–1012. doi: 10.1126/science.2343305. [DOI] [PubMed] [Google Scholar]
- Ross R. The fibroblast and wound repair. Biol Rev Camb Philos Soc. 1968 Feb;43(1):51–96. doi: 10.1111/j.1469-185x.1968.tb01109.x. [DOI] [PubMed] [Google Scholar]
- Rönnstrand L., Terracio L., Claesson-Welsh L., Heldin C. H., Rubin K. Characterization of two monoclonal antibodies reactive with the external domain of the platelet-derived growth factor receptor. J Biol Chem. 1988 Jul 25;263(21):10429–10435. [PubMed] [Google Scholar]
- Shaw R. J., Doherty D. E., Ritter A. G., Benedict S. H., Clark R. A. Adherence-dependent increase in human monocyte PDGF(B) mRNA is associated with increases in c-fos, c-jun, and EGR2 mRNA. J Cell Biol. 1990 Nov;111(5 Pt 1):2139–2148. doi: 10.1083/jcb.111.5.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shure D., Senior R. M., Griffin G. L., Deuel T. F. PDGF AA homodimers are potent chemoattractants for fibroblasts and neutrophils, and for monocytes activated by lymphocytes or cytokines. Biochem Biophys Res Commun. 1992 Aug 14;186(3):1510–1514. doi: 10.1016/s0006-291x(05)81577-3. [DOI] [PubMed] [Google Scholar]
- Soma Y., Dvonch V., Grotendorst G. R. Platelet-derived growth factor AA homodimer is the predominant isoform in human platelets and acute human wound fluid. FASEB J. 1992 Aug;6(11):2996–3001. doi: 10.1096/fasebj.6.11.1644262. [DOI] [PubMed] [Google Scholar]
- Sprugel K. H., McPherson J. M., Clowes A. W., Ross R. Effects of growth factors in vivo. I. Cell ingrowth into porous subcutaneous chambers. Am J Pathol. 1987 Dec;129(3):601–613. [PMC free article] [PubMed] [Google Scholar]
- Sundberg C., Ljungström M., Lindmark G., Gerdin B., Rubin K. Microvascular pericytes express platelet-derived growth factor-beta receptors in human healing wounds and colorectal adenocarcinoma. Am J Pathol. 1993 Nov;143(5):1377–1388. [PMC free article] [PubMed] [Google Scholar]
- Sánchez A., Chesterman C. N., Sleigh M. J. Novel human PDGFA gene transcripts derived by alternative mRNA splicing. Gene. 1991 Feb 15;98(2):295–298. doi: 10.1016/0378-1119(91)90189-i. [DOI] [PubMed] [Google Scholar]
- Terracio L., Rönnstrand L., Tingström A., Rubin K., Claesson-Welsh L., Funa K., Heldin C. H. Induction of platelet-derived growth factor receptor expression in smooth muscle cells and fibroblasts upon tissue culturing. J Cell Biol. 1988 Nov;107(5):1947–1957. doi: 10.1083/jcb.107.5.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong B. D., Auer D. E., Jaye M., Kaplow J. M., Ricca G., McConathy E., Drohan W., Deuel T. F. cDNA clones reveal differences between human glial and endothelial cell platelet-derived growth factor A-chains. Nature. 1987 Aug 13;328(6131):619–621. doi: 10.1038/328619a0. [DOI] [PubMed] [Google Scholar]
- Yeh H. J., Pierce G. F., Deuel T. F. Ultrastructural localization of a platelet-derived growth factor/v-sis-related protein(s) in cytoplasm and nucleus of simian sarcoma virus-transformed cells. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2317–2321. doi: 10.1073/pnas.84.8.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. M., Mendoza A. E., Collins T., Orkin S. H. Alternatively spliced platelet-derived growth factor A-chain transcripts are not tumor specific but encode normal cellular proteins. Mol Cell Biol. 1990 Nov;10(11):6051–6054. doi: 10.1128/mcb.10.11.6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeder-Lutz G., Altschuh D., Geysen H. M., Trifilieff E., Sommermeyer G., Van Regenmortel M. H. Monoclonal antipeptide antibodies: affinity and kinetic rate constants measured for the peptide and the cognate protein using a biosensor technology. Mol Immunol. 1993 Feb;30(2):145–155. doi: 10.1016/0161-5890(93)90086-q. [DOI] [PubMed] [Google Scholar]