Abstract
Aim
To assess the short‐term efficacy of hypotonic 0.18% sodium hyaluronate in patients with evaporative tear‐sufficient dry eye due to lipid tear deficiency (LTD).
Methods
This was a randomised, double‐blind, controlled, exploratory study. A total of 10 patients with dry eye due to LTD were treated as follows: one drop of hypotonic 0.18% sodium hyaluronate in one eye and one drop of isotonic 0.3% hydroxypropyl‐methylcellulose (HPMC)/0.1% dextran in the other eye. Non‐invasive tear film break‐up time (NIBUT) evaluated by using a tear scope with grid pattern and subjective ocular symptoms of dry eye were assessed at 15, 30, 60 and 90 min after instillation.
Results
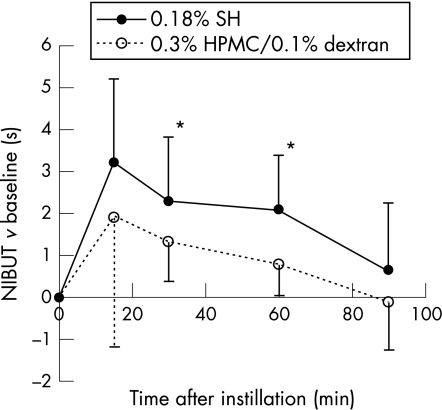
Both sodium hyaluronate and HPMC/dextran caused a significant (p<0.05) improvement in NIBUT and symptoms. Mean (SD) NIBUT in the sodium hyaluronate group was 3.2 (1.0), 6.4 (2.8), 5.5 (1.9), 5.3 (1.3) and 3.9 (1.7) s at 0, 15, 30, 60 and 90 min, respectively, compared with 3.6 (1.9), 5.5 (3.2), 5.0 (1.5), 4.4 (2.2) and 3.5 (1.2) s in the HPMC/dextran group. However, increase in NIBUT was significantly (p<0.05) greater and longer in the sodium hyaluronate group than in the HPMC/dextran group.
Conclusion
Treatment with sodium hyaluronate and HPMC/dextran eye drops is useful for treating patients with dry eye due to LTD. However, sodium hyaluronate caused a significantly (p<0.05) greater increase in NIBUT values than HPMC/dextran in such patients.
Causes of dry eye have been classified into two groups—namely, aqueous tear‐deficient dry eye (ATD) and tear‐sufficient evaporative dry eye.1 Artificial tears are widely used to treat ATD. In most severe cases, treatment with non‐preserved eye drops is mandatory to avoid the toxicity of preservative when using the eye drops at frequent intervals. The efficacy of non‐preserved artificial tears is well recognised in this case.2
Sodium hyaluronate as a tear‐replacement eye drop is widely used in the treatment of ATD, as indicated in several well‐controlled studies.3,4,5 However, little is known on the efficacy profile of sodium hyaluronate in evaporative dry eye due to lipid tear deficiency (LTD). LTD is one of the important causes of tear film instability. It is caused by meibomian gland abnormality, which results in lack of or abnormal lipid tear layer, instability of the tear film and shortening of tear break‐up time (TBUT).2,6
As a consequence, the purpose of this study was to assess the performance profile of hypotonic 0.18% sodium hyaluronate eye drops after a single instillation in patients with dry eye due to LTD. Hydroxypropyl‐methylcellulose (HPMC)/dextran eye drops were used as the reference product.
Materials and methods
Study design
This was a randomised, double‐blind, controlled, exploratory study. Before initiation of the study, the informed consent form and protocol were approved by the committee for the protection of human participants in research at the Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok, Thailand. The study was conducted in accordance with good clinical practice guidelines for the evaluation of medicinal products and the Declaration of Helsinki. At the day 0 visit, patients were checked for inclusion and exclusion criteria, and the following baseline assessments for parameters were performed: dry eye questionnaire, slit lamp examination, non‐invasive tear film break‐up time (NIBUT) using a Tearscope and TBUT using fluorescein, corneal staining with fluorescein, staining with rose Bengal and tear volume (Schirmer I test). Tear film instability can be best shown by NIBUT using a tear scope with a grid pattern. The non‐contact nature of the tear scope without any substrate put into the eye allows us to evaluate the tear film in the natural environment.
Thereafter, at day 1, eligible patients were randomly allocated to receive one drop of sodium hyaluronate in one eye, whereas the other eye received one drop of HPMC/dextran. Another person instilled the assigned product in accordance with the randomisation table so that the assessor was blinded to the treatment received.
NIBUT and symptoms were then assessed at 15, 30, 60 and 90 min after instillation.
Inclusion and exclusion criteria
A total of 10 patients with evaporative lipid‐deficient dry eye syndrome due to pure LTD according to the definition of Lemp1 were enrolled in the study. Inclusion criteria were patients aged ⩾18 years, with meibomian gland dysfunction (MGD), with NIBUT<10 s in each eye, Schirmer's I test>10 mm wetting/5 min in each eye and at least one of the following symptoms of dry eye: soreness, scratchiness, dryness, grittiness and burning. Exclusion criteria were severe dry eye, ocular surgery within the past 4 months before inclusion, use of preserved eye drops within the past 2 weeks before inclusion, wearing contact lens, abnormality of the nasolacrimal drainage apparatus and hypersensitivity to hyaluronic acid or any component or, excipient used in the study.
Clinical assessments in each eye
The intensity of five dry eye symptoms (included soreness, scratchiness, dryness, grittiness and burning, as described in the McMonnies questionnaire7) was assessed on five separate 0 mm (no symptom) to 100 mm (severe symptom) visual analogue scales.8 An average symptom score (maximum 100 mm) was calculated. Ocular examinations included visual acuity, external and slit lamp examination on lid, lashes and meibomian glands, staining with fluorescein and rose Bengal, NIBUT, TBUT and tear volume (Schirmer's I test).
Meibomian gland abnormality was defined as one or more of the following criteria: abnormal secretion, abnormal expressibility and squamous metaplasia of the meibomian gland orifice.
Rose Bengal was assessed using the 0–9 scoring system of Bijsterveld.9 The fluorescein staining scoring system (score 0–12) was calculated using type, extent and depth of corneal erosion (type: micropunctate = 1, macropunctate = 2, coalescence = 3 and patch = 4; extent (percentage of surface area): 1–15% = 1, 16–30% = 2, 31–45% = 3 and >45% = 4; and depth: superficial epithelial punctate = 1, deep delayed epithelial punctate = 2, immediate localised stromal glow = 3 and immediate diffuse stromal glow = 4).
NIBUT was measured using the Tearscope Plus (Keeler, Windsor, UK).10,11 The assessor (PP) measured the interval between a complete blink and the appearance of the first randomly distorted grid pattern on the corneal tear film, and calculated the average value of three measurements. This test was performed before any eye drops or dye staining was instilled into the eye.
Tear volume (Schirmer's I test) was measured over a 5‐min period without anaesthetic drops.
Ophthalmic examination and reporting of adverse event throughout the study were assessed as regards safety. All the examinations and tests were recorded by the same assessor (PP). Both the assessor and patients were blinded to the treatment groups (double blind).
Products
Commercially available study products—namely, Vislube/Vismed (0.18% sodium hyaluronate, molecular weight 1.2×106 Da, TRB Chemedica, Munich, Germany) and BionTears (0.3% HPMC/0.1% dextran, Alcon, Forth Worth, Texas, USA) were used. Vislube/Vismed is a patented hypotonic (150 mOsm/kg) solution containing the electrolytes potassium, calcium, magnesium, sodium and chloride. BionTears is an isotonic solution containing sodium, potassium, calcium, magnesium, zinc, chloride and bicarbonate as electrolytes. Both products are preservative free.
Statistics
As this was an exploratory trial, no prior hypothesis was chosen and no sample size was calculated. No primary efficacy parameter was chosen. However, NIBUT was considered to be the most important assessment parameter in this particular trial.
Comparisons between groups were performed using Student's t test (5% level). Homogeneity between groups at baseline was tested using Student's t test (10% level).
Results
Ten patients (9 women and 1 men; 20 eyes) were included and completed the study in accordance with the protocol. Mean (standard deviation (SD)) age was 45.9 (16.4) years (median 39.5, range 25–70 years).
Table 1 shows that the two treatment groups were homogeneous for demographic characteristics. Both groups had only minimal fluorescein and rose Bengal staining. Half of the patients in both groups had minimal superficial punctate fluorescein staining (<15% surface area), and two and three patients in the sodium hyaluronate and HPMC groups, respectively, had minimal rose Bengal staining (score 1–2).
Table 1 Characteristics of patients at baseline (n = 10).
| Characteristic | Treatment group (mean (SD)) | |
|---|---|---|
| 0.18% SH | HPMC/dextran | |
| NIBUT (s) | 4.1 (1.6) | 3.9 (2.1) |
| FBUT (s) | 3.4 (1.3) | 3.5 (1.9) |
| Corneal staining with fluorescein (score) | 1.5 (1.6) | 1.5 (1.6) |
| Staining with rose Bengal (score) | 0.4 (0.8) | 0.4 (0.7) |
| Tear volume (Schirmer's I test; mm) | 28.9 (16.9) | 27.6 (15.4) |
FBUT, fluorescein tear break‐up time; HPMC, hydroxypropyl‐methylcellulose; NIBUT, non‐invasive tear film break‐up time; SH, sodium hyaluronate.
After a single instillation of artificial tear, mean (SD) NIBUT in the sodium hyaluronate group was 3.2 (1.0), 6.4 (2.8), 5.5 (1.9), 5.3 (1.3) and 3.9 (1.7) s at 0, 15, 30, 60 and 90 min compared with 3.6 (1.9), 5.5 (3.2), 5.0 (1.5), 4.4 (2.2) and 3.5 (1.2)s in the HPMC/dextran group, respectively. Both sodium hyaluronate and HPMC/dextran caused a significant (p<0.05) improvement in NIBUT (fig 1) at 15, 30 and 60 min compared with the baseline. However, improvement observed in the sodium hyaluronate group was greater at all time points and longer (>90 min) than that in the HPMC group (60 min). Differences were significant at 30 min (p = 0.04) and 60 min (p = 0.005) in favour of sodium hyaluronate and close to significance at 15 min (p = 0.08) and 90 min (p = 0.07).
Figure 1 Results of non‐invasive break‐up time (NIBUT; difference from baseline, mean (SD), in seconds) after a single instillation of 0.18% sodium hyaluronate (SH) in one eye and 0.3% hydroxypropyl‐methylcellulose (HPMC)/0.1% dextran in the other eye. *p<0.05 sodium hyaluronate versus HPMC/dextran.
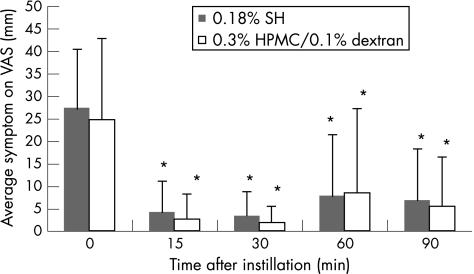
Looking at symptoms on the visual analogue scale (fig 2), a significant (p<0.05) improvement was seen in both groups at all time points. However, no significant difference between the two groups was observed for this parameter. Each symptom separately (ie, soreness, scratchiness, dryness, grittiness and burning) showed a profile of improvement similar to that of average symptoms.
Figure 2 Results of symptoms on the visual analogue scale (VAS) (mean (SD), in mm) after a single instillation of 0.18% sodium hyaluronate (SH) in one eye and 0.3% hydroxypropyl‐methylcellulose (HPMC)/0.1% dextran in the other eye. *p<0.05 versus baseline.
No adverse event in any group was observed by the investigator or reported by the patient throughout the study. Both treatments were well tolerated and did not affect visual acuity except temporally blurred vision immediately after instillation.
Discussion
Dry eye was defined by the National Eye Institute/Industry Workshop as a disorder of the tear film due to tear deficiency or excessive evaporation.1 ATD can be caused by deficiency of aqueous production from the lacrimal gland resulting from an inflammatory process such as Sjögren's syndrome or decrease in corneal sensitivity due to many reasons including ageing, a refractive surgical procedure or systemic diseases.2 Evaporative tear‐sufficient dry eye can be caused by many factors such as increased exposure, decrease in blinking, anatomical abnormality, decrease in corneal sensitivity and MGD.2 MGD causes abnormal lipid tear layers, leading to instability of the tear film12,13 and abnormal tear film spreading.14 This induces ocular surface abnormality, which may result in ocular surface damage6 and ocular discomfort such as a burning sensation, irritation and blurry vision. Dry eye due to LTD can be diagnosed by rapid TBUT, whereas aqueous production remains normal.2 In this study, we included such patients, on the basis of short TBUT values (<10 s) and normal values of tear volume (Schirmer's I test>10 mm wetting/5 min), to assess whether sodium hyaluronate and HPMC/dextran could increase tear film stability and relieve the symptoms.
Tear film break‐up time was determined using a non‐invasive technique, thus avoiding the instillation of fluorescein, as fluorescein itself has been shown to shorten the TBUT15,16 and modify the effect of the sodium hyaluronate solution being instilled.17 In this study, we also showed that TBUT values were lower than those obtained with the non‐invasive technique (NIBUT), where no fluorescein dye was used at baseline (table 1).
Previous reports of sodium hyaluronate in ATD3,4,5,18 yielded excellent results compared with reference treatments or saline. Hypotonic sodium hyaluronate solutions were shown to further improve the outcome in such patients compared with normal isotonic eye drops.3 Sodium hyaluronate (molecular weight 1×106 Da) at a concentration of 0.1% was also shown to significantly increase the tear break‐up time in patients with dry eye and in the normal population compared with saline or sodium hyaluronate at lower concentrations.15,19 These findings suggest that sodium hyaluronate at a concentration of at least 0.1% is required to delay the break‐up of the precorneal tear film in patients with ATD.
In this study, we showned that a single topical application of 0.18% sodium hyaluronate and HPMC/dextran also significantly improved tear film stability in patients with tear dysfunction due to lipid deficit, although these patients have normal tear volume. Moreover, 0.18% sodium hyaluronate caused a significantly prolonged TBUT compared with HPMC/dextran for up to 90 min after instillation of a single drop. This result might be explained by the peculiar properties of sodium hyaluronate. Sodium hyaluronate is a biopolymer that occurs naturally in all vertebrates—for example, in the vitreous body of the eye, the extracellular matrix of the skin and in the synovial fluid. It has unique characteristics that make it ideal to improve tear film stability, including viscoelastic rheological behaviour,20 mucomimetic properties,21 water‐retention properties22 and the ability to delay evaporation of the aqueous component of the tear film (water‐retention properties).
Owing to the increased aqueous tear evaporation in lipid tear dysfunction,23,24 tear osmolarity in LTD and MGD was reported to be higher than normal.23,25 This suggests that hypotonic sodium hyaluronate eye drops may be superior to isotonic eye drops in patients with MGD or LTD.
In general, treatment of LTD is more difficult than ATD because artificial lipid‐replacement therapy is not currently available. Treatment strategies include the attempt to improve the function of the meibomian gland using warm compression, oral tetracycline13 and emulsion eye drops.14,26,27,28 In this study, we showed that non‐preserved aqueous sodium hyaluronate and HPMC/dextran solutions significantly improved the tear stability and symptoms of patients with LTD, with significantly better findings in the sodium hyaluronate group. This finding may be useful in clinical practice because these non‐preserved artificial tears are commercially available.
In an earlier study on patients with ATD, Mengher et al15 reported an improvement in TBUT for 40 min after a single instillation of one drop of a 0.1% sodium hyaluronate solution. The longer duration of effect (90 min) observed in our study can be attributed to the higher concentration of sodium hyaluronate used (0.18% v 0.1%), and also to the different type of dry eye treated. Although 0.18% sodium hyaluronate gave more favourable results than HPMC/dextran in our study, the results indicated that the generally recommended treatment regimen of 3–4 instillations per day is not sufficient to provide the patient with adequate relief. These patients should receive treatment at least every 1 or 2 h before the effect becomes evident.
In this study, the symptoms of dry eye were immediately and significantly alleviated for up to 90 min after instillation in both groups. However, the efficacy tended to reduce after 60 min. This result is similar to that obtained by Mengher et al15 in patients with ATD. There was no burning sensation or serious complication arising from both non‐preserved eye drops.
In conclusion, both sodium hyaluronate and HPMC/dextran are beneficial not only to patients with ATD for increasing tear volume but also to patients with dry eye due to LTD because they improve tear film stability and symptoms. In this trial, sodium hyaluronate caused considerably better relief than HPMC/dextran in such patients. However, the results would need to be confirmed in another study with a larger number of patients to further assess the long‐term benefits of non‐preserved eye drops in this indication.
Acknowledgements
We thank Dr Vincent Baeyens for his advice and support during this study.
Abbreviations
ATD - aqueous tear‐deficient dry eye
HPMC - hydroxypropyl‐methylcellulose
LTD - lipid tear deficiency
MGD - meibomian gland dysfunction
NIBUT - non‐invasive tear film break‐up time
TBUT - tear break‐up time
Footnotes
Competing interests: None declared.
References
- 1.Lemp M A. Report of the National Eye Institute/Industry Workshop on clinical trials in dry eyes. CLAO J 199521221–232. [PubMed] [Google Scholar]
- 2.Djalilian A R, Hamrah P, Pflugfelder S C. In: Krashmer JH, Mannis MJ, Holland EJ, eds. Cornea. 2nd edn. Philadelphia: Elsevier, 2005521–540.
- 3.Aragona P, Di Stefano G, Ferreri F.et al Sodium hyaluronate eye drops of different osmolarity for the treatment of dry eye in Sjogren's syndrome patients. Br J Ophthalmol 200286879–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aragona P, Papa V, Micali A.et al Long term treatment with sodium hyaluronate‐containing artificial tears reduces ocular surface damage in patients with dry eye. Br J Ophthalmol 200286181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brignole F, Pisella P J, Dupas B.et al Efficacy and safety of 0.18% sodium hyaluronate in patients with moderate dry eye syndrome and superficial keratitis. Graefes Arch Clin Exp Ophthalmol 2005243531–538. [DOI] [PubMed] [Google Scholar]
- 6.Lee S ‐ H, Tseng S C G. Rose bengal staining and cytologic characteristics associated with lipid tear deficiency. Am J Ophthalmol 1997124736–750. [DOI] [PubMed] [Google Scholar]
- 7.McMonnies C W. Key questions in a dry eye history. J Am Optom Assoc 198657512–517. [PubMed] [Google Scholar]
- 8.McMonnies C W, Ho A. Patient history in screening for dry eye conditions. J Am Optom Assoc 198758296–301. [PubMed] [Google Scholar]
- 9.Bijsterveld O P. Diagnostic tests in the Sicca syndrome. Arch Ophthalmol 19698210–14. [DOI] [PubMed] [Google Scholar]
- 10.Guillon J P. The Keeler Tearscope‐Plus ‐ an improved device for assessing the tear film. Optician 19972131–4. [Google Scholar]
- 11.Guillon M, Guillon J. Tear film examination of contact lens patient. Optician 199320621–29. [Google Scholar]
- 12.Rolando M, Refojo M F, Kenyon K R. Tear water evaporation and eye surface diseases. Ophthalmologica 1985190147–149. [DOI] [PubMed] [Google Scholar]
- 13.Zengin N, Tol H, Gunduz K.et al Meibomian gland dysfunction tear film abnormalities in rosacea. Cornea 199514144–146. [PubMed] [Google Scholar]
- 14.Goto E, Tseng S C. Differentiation of lipid tear deficiency dry eye by kinetic analysis of tear interference images. Arch Ophthalmol 2003121173–180. [DOI] [PubMed] [Google Scholar]
- 15.Mengher L S, Pandher K S, Bron A J.et al Effect of sodium hyaluronate (0.1%) on break‐up time (NIBUT) in patients with dry eyes. Br J Ophthalmol 198670442–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mengher L S, Bron A J, Tonge S R.et al A non‐invasive instrument for clinical assessment of the precorneal tear film stability. Curr Eye Res 198541–7. [DOI] [PubMed] [Google Scholar]
- 17.Golding T R, Efron N, Brennan N A. Soft lens lubricants and prelens tear film stability. Optom Vis Sci 199067461–465. [DOI] [PubMed] [Google Scholar]
- 18.Condon P I, McEwen C G, Wright M.et al Double blind, randomised, placebo controlled, crossover, multicentre study to determine the efficacy of a 0.1% (w/v) sodium hyaluronate solution (Fermavisc) in the treatment of dry eye syndrome. Br J Ophthalmol 1999831121–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson M E, Murphy P J, Boulton M. Effectiveness of sodium hyaluronate eyedrops in the treatment of dry eye. Graefes Arch Clin Exp Ophthalmol 2005281–4. [DOI] [PubMed] [Google Scholar]
- 20.Tiffany J M. Viscoelastic properties of human tears and polymer solutions. Adv Exp Med Biol 1994350267–270. [DOI] [PubMed] [Google Scholar]
- 21.Tiffany J M, Winter N, Bliss G. Tear film stability and tear surface tension. Curr Eye Res 19898507–515. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura M, Hikida M, Nakano T.et al Characterization of water retentive properties of hyluronan. Cornea 199312433–436. [DOI] [PubMed] [Google Scholar]
- 23.Mathers W D, Binarao G, Petroll M. Ocular water evaporation and the dry eye. A new measuring device. Cornea 199312335–340. [DOI] [PubMed] [Google Scholar]
- 24.Mathers W D. Ocular evaporation in meibomian gland dysfunction and dry eye. Ophthalmology 199334751–100. [DOI] [PubMed] [Google Scholar]
- 25.Mathers W D. Meibomian gland morphology and tear osmolarity: changes with Accutane therapy. Cornea 199110286–290. [DOI] [PubMed] [Google Scholar]
- 26.Goto E, Shimazaki J, Monden Y.et al Low‐concentration homogenized castor oil eye drops for noninflamed obstructive meibomian gland dysfunction. Ophthalmology 20021092030–2035. [DOI] [PubMed] [Google Scholar]
- 27.Di Pascuale M A, Goto E, Tseng S C G. Sequential changes of lipid tear film after the instillation of a single drop of a new emulsion eye drop in dry eye patients. Ophthalmology 2004111783–791. [DOI] [PubMed] [Google Scholar]
- 28.Korb D R, Scaffidi R C, Greiner J V.et al The effect of two novel lubricant eye drops on tear film lipid layer thickness in subjects with dry eye symptoms. Optom Vis Sci 200528594–601. [DOI] [PubMed] [Google Scholar]