Abstract
Phantom limbs are traditionally conceptualized as the phenomenal persistence of a body part after deafferentation. Previous clinical observations of subjects with phantoms of congenitally absent limbs are not compatible with this view, but, in the absence of experimental work, the neural basis of such “aplasic phantoms” has remained enigmatic. In this paper, we report a series of behavioral, imaging, and neurophysiological experiments with a university-educated woman born without forearms and legs, who experiences vivid phantom sensations of all four limbs. Visuokinesthetic integration of tachistoscopically presented drawings of hands and feet indicated an intact somatic representation of these body parts. Functional magnetic resonance imaging of phantom hand movements showed no activation of primary sensorimotor areas, but of premotor and parietal cortex bilaterally. Movements of the existing upper arms produced activation expanding into the hand territories deprived of afferences and efferences. Transcranial magnetic stimulation of the sensorimotor cortex consistently elicited phantom sensations in the contralateral fingers and hand. In addition, premotor and parietal stimulation evoked similar phantom sensations, albeit in the absence of motor evoked potentials in the stump. These data indicate that body parts that have never been physically developed can be represented in sensory and motor cortical areas. Both genetic and epigenetic factors, such as the habitual observation of other people moving their limbs, may contribute to the conscious experience of aplasic phantoms.
The experience of a phantom limb is universal among amputees and frequent after deafferentation of an extremity at the level of the spinal cord (1). The common explanation for the phantom limb phenomenon is in terms of a central representation that survives peripheral loss, i.e., phantom sensations are considered as perceptuomotor “memories” of a once functional body part. This view is challenged, however, by clinical reports on subjects with aplasic phantoms, i.e., phantom sensations of congenitally absent limbs (2–9). Such case studies have appeared periodically in the medical literature for well over 150 years, but, to the best of our knowledge, no attempts have ever been undertaken to elucidate the neural basis of aplasic phantoms by systematic experimentation.
We report in this paper the results of an extensive examination of a person with aplasic phantoms. Behavioral assessment included a well-established task that requires speeded left/right decisions for visually presented hands and feet (10–13). Chronometric studies indicated that normal subjects solve this “implicit reaching” task by mentally moving a stored representation of their own hand/foot into the portrayed position (10, 11). Consequently, subjects' correct decisions are substantially slower for stimuli in which the fingers/toes point down rather than up. This task would allow us to decide between two mutually exclusive interpretations of aplasic phantoms. If our subject's phantom hands and feet were the mere products of wishful thinking that lacked a proper somatic representation (14), we would expect absence of a rotation effect. Presence of a rotation effect, on the other hand, would point to a somatic representation of hands and feet whose kinematic properties were subject to the natural biomechanical joint constraints (11). Similar psychophysical procedures previously have substantiated the subjective experience of phantom movements in traumatic amputees (see example in ref. 15).
To identify potential cortical areas activated during voluntary movements of our subject's, A.Z.'s, phantom body parts, we studied brain activation patterns by using functional magnetic resonance imaging (fMRI) during those phantom limb movements to which the subject had previously ascribed a particularly high vividness. We also tested the pattern of cortical activation when A. Z. was moving intact parts of her body. The rationale was to determine whether the cortical areas for hand and finger representations would be activated by movements of existing body parts with neighboring somatotopical representations. Various techniques previously have revealed such expansions in traumatic amputees (1).
Complementary to the fMRI study, we mapped A.Z.'s sensorimotor cortex by transcranial magnetic stimulation (TMS). Motor evoked potentials (MEPs) were acquired from deltoid muscles while we protocolled the subject's introspective report concerning any sensations in stumps, phantom limbs, or both.
Before turning to these experimental procedures in more detail, we report the clinical features of our subject, emphasizing the phenomenology of her phantom sensations. A.Z. is a 44-year-old university-educated woman born without forearms and legs. The reasons for her tetra-amelia are unknown. She has two healthy sisters, and there is no family history of limb-reduction defects. Apart from a pronounced spinal scoliosis, A.Z. shows no other physical anomalies. Her upper arms consist of two conical stumps ≈25 cm long and freely movable in the shoulder joint. X-ray pictures show normal shoulder articulations with proximal humeri without elbow articulations. A.Z. does not wear a prosthesis, but skillfully grasps objects and is able to typewrite with the tips of her stumps. She eats by herself with the aid of a fork attached to a ring placed on her right upper arm. She writes with her mouth. A.Z.'s thighs measure ≈10 cm, and the x-rays show rudimentary femurs and dysplasic hip articulations bilaterally. She uses an electric wheelchair, which she steers with her right upper stump.
For as long as A.Z. can remember, mental images of forearms (including hands and fingers) and legs (with feet and first and fifth toes) have been experienced as integral parts of her own body. Awareness of her phantom limbs is transiently disrupted only when some object or person invades their felt position or when she sees herself in a mirror. While manipulating objects with an arm stump, the subject usually feels the phantom fingers attached to the stump, and she is no longer aware of a forearm. As soon as contact with the object is lost, the fingers immediately switch back to their regular distal position. A.Z. spontaneously reported that the vividness of her phantom foot/leg sensations was occasionally enhanced during stimulation of the genital area. This phenomenon of “referred sensations” has been described in amputees and interpreted in terms of the topology of the cortical somatosensory map (16). However, on being touched in the face or on the upper arms, A.Z. has never experienced simultaneous sensations in her phantom upper extremities, and standard methods of stimulation (17, 18) did not produce such referred sensations.
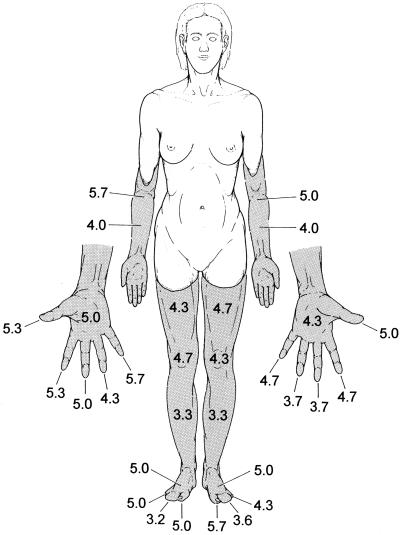
A structured interview asking for vividness ratings (on a 7-point scale) of various aspects of phantom sensations was administered on three different days separated by ≈3) weeks. With a high consistency across sessions, awareness of position (Fig. 1) and ability to move phantom body parts received the highest vividness ratings.
Figure 1.
Phantoms (shaded areas) in a subject with limb amelia. The numbers are vividness ratings (means of three measurements) for the felt presence of different phantom body parts on a 7-point scale from 0 (no awareness) to 6 (most vivid impression).
Pain and temperature sensations were rated as absent. Catch-items, controlling for suggestibility (e.g., “In darkness, I have noted a faint glowing of my phantom body parts.”) consistently received zero ratings (i.e., “never experienced”). To determine the hand and finger phantom movements optimally suited for the fMRI experiments, similar vividness ratings also were obtained for dynamic aspects of A.Z.'s phantom sensations. In three different sessions, phantom finger-to-thumb opposition consistently received comparatively high ratings (3, 4, 5 and 3, 3, 5 for the left and right hands, respectively) as did pronation-supination of the left and right wrists (6, 5, 5 and 5, 5, 5, respectively). Conversely, ulnar-radial alternation of the phantom wrists was difficult to achieve (3, 0, 2 and 2, 1, 2 for the left and right wrists, respectively). Vividness of flexion-extension of the right and left phantom feet received ratings of 5 in each of the three sessions.
Methods
All experiments had been approved by the Ethical Committee of the University Hospital Zürich, and the subject gave written informed consent before participation.
Implicit Reaching Task.
In the center of a computer screen, a randomized sequence of drawings of eight different hands and two different feet was shown to A.Z. (see Fig. 2 for sample stimuli). With her right stump, she had to press the space bar of a keyboard whenever a right limb was displayed (4 runs with 40 stimulations each) or, conversely, with her left stump whenever a left limb was displayed (4 additional runs). Right-limb target runs and left-limb target runs were presented in an alternate sequence in two different sessions separated by ≈3 weeks. Within each run, half of the stimuli portrayed a left and half a right limb, i.e., each limb was shown also as its exact mirror version. The two finger and toe positions (up vs. down; mirror-reversals in the horizontal plane) were shown with equal frequencies. Stimuli extended ≈8.5° of visual angle horizontally and 11° vertically. They were displayed for 500 ms.
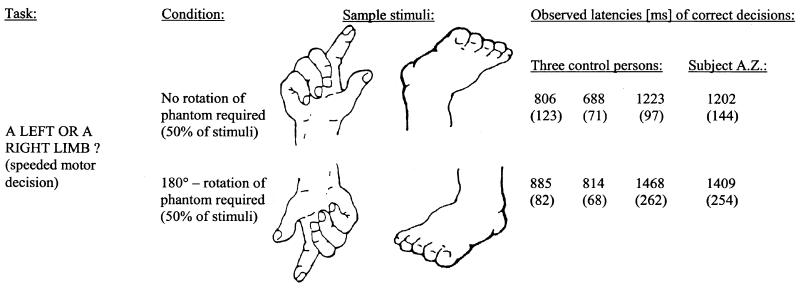
Figure 2.
Implicit reaching task. Displayed are task demands and conditions of stimulus presentation along with two sample stimuli for each condition. Mean latencies (SD in brackets) of correct decisions for subject A.Z. and three control subjects also are listed. In all subjects, mental rotation of a limb during implicit reaching was reflected by significantly longer decision times.
fMRI.
Echoplanar magnetic resonance brain images were acquired on a 1.5-T Signa System (General Electric) equipped with an ultrafast three-axis gradient system, using a circularly polarized head coil. fMRIs were obtained in a horizontal plane by using a blood oxygenation level-dependent (BOLD in ref. 19) acquisition (gradient-echo, T2*-weighted, single-shot echo planar imaging: repetition time = 3 s; echo time = 40 ms; flip angle = 40°) with an in-plane resolution of 3.1 mm × 3.1 mm. Seven contiguous 5-mm thick slices covering the entire primary sensorimotor, premotor, and superior parietal cortex were obtained. Forty images were acquired, interleaved in each imaging plane during five alternating periods of rest and task performance (5–10-10–10-5 images in each period, respectively) for a total of 280 images. T1-weighted, spin-echo images were taken in register with each functional scan plane for anatomical localization of functional activity. The subject's head was stabilized by restraints and cushioning. Functional images were realigned for each experiment by using an automated image registration algorithm (20). During the functional experiments, the subject was instructed to keep her eyes closed. To build nonparametric maps, voxels activated during the task conditions were identified by calculating Spearman rank-order correlation coefficients between the time series of MRI intensities in a single voxel and an idealized response function, and transformation to Student's t test statistics were made. Those pixels with statistically significant correlation coefficients according to the Student's t test (P < 0.001) were considered as activated areas and are referred to in the text as “activation.” Functional maps were overlaid directly onto the corresponding T1-weighted anatomical MRIs obtained in the same session. Tasks of phantom limb movements included: (i) self-paced sequential finger-to-thumb opposition of the right phantom fingers; (ii) the same movements as in i for the left phantom fingers; (iii) pronation-supination of the right phantom hand; and (iv) flexion-extension of the right phantom foot. The movements required for testing the reorganization in the region of the primary motor representation of the hand (M1; ref. 21) involved: (i) flexion-extension of the right upper stump; (ii) flexion-extension of the left upper stump; (iii) kissing movements with the lips; (iv) eye blinking; and (v) lateral movements of the tongue. For the foot representation, flexion-extension movements of the right lower stump were investigated. All movement sequences, whether of phantoms or of existing body parts, were self-paced in a constant rhythm of approximately one cycle per second. They had been practiced by the subject before the first fMRI session. Originally, we planned to compare “executed” with “imagined” phantom movements rather than with periods of rest. This plan was abandoned after practice sessions revealed that these two tasks were phenomenologically identical for A.Z. Repeated electromyographic recordings from the upper arm (deltoid), performed outside the scanner, indicated complete absence of stump muscle activation during movements of the phantom wrist, hand, and fingers. All fMRI scanning was performed in two imaging sessions separated by <3 weeks.
TMS.
During TMS, the subject lay supine on a comfortable bed in a quiet room. Surface electromyography (EMG) was recorded from right and left deltoid muscles, which both had a normal anatomical appearance. The signals were stored in a laboratory computer (Medelec MYSTRO, NBN Electronics, Uitikon, Switzerland) for offline analysis. Muscle relaxation was monitored by visual and acoustical feedback of the EMG. A snugly fitting cap was positioned over the subject's head, and the vertex (Cz) was identified as central reference mark according to the 10–20 system. For TMS mapping, a 2 by 2-cm grid was established which originated from Cz. TMS was delivered by a standard figure-of-eight coil [Magstim 200; Magstim Company, Spring Gardens, Whitland (Dyfed), U.K.] in one single session. First, the optimal scalp position for eliciting maximal MEPs from the deltoid muscle was localized. Then, the motor threshold was determined at complete rest, monitored by EMG, to the nearest 1% of the stimulator output. Finally, the experiment was performed with the magnetic coil positioned with its handle oriented posteriorly at 110% of the motor threshold. The motor threshold of the deltoid muscle for the optimal cortical position was 51% on the right side and 61% on the left. The criterion for the existence of an MEP was a deflection in the EMG of at least 0.1 mV with an appropriate latency. At least two stimulations were delivered at each of the 65 stimulated grid points. Centers of gravity of MEP amplitude for the deltoid muscles were calculated according to ref. 22. They had the following coordinates with reference to Cz: 3.6 cm lateral, 0.5 cm anterior (right hemisphere); and 5.7 cm lateral, 1.5 cm anterior (left hemisphere). These coordinates were within the 95% confidence interval of control subjects (22). The mean amplitude of the MEP was 13.0 mV (SD = 0.67 mV), and the mean latency was 12.5 ms (SD = 1.12 ms). Subject's report of the sensations elicited by TMS was protocolled verbatim.
Results
Implicit Reaching Task.
A.Z.'s reaction times and performance accuracy (81.9% correct discriminations) were within the range of three age- and education-matched female control subjects performing the same task (93.8%, 83.8%, and 64.4%, respectively). More importantly, A.Z.'s correct decisions were significantly faster when fingers or toes pointed up than when the limbs were portrayed under 180° rotation (t = 2.7; P < 0.05, two-tailed; Fig. 2). This pattern was also observed for each of the three control persons (2.1 < t < 6.4; 0.001 < Ptwo-tailed < 0.05).
fMRI of Phantom Movements.
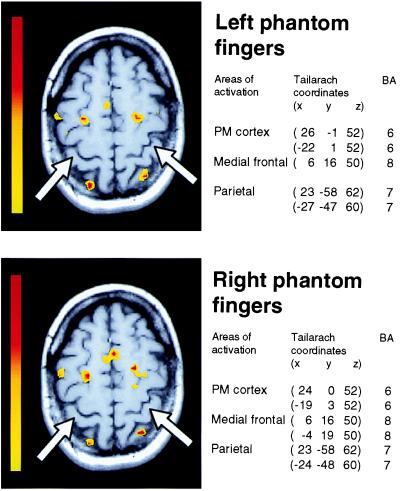
Compared with a condition of rest (no phantom movements), phantom finger movements produced consistent bilateral activation of the dorsal premotor cortex at the junction of the superior frontal and precentral sulci. Further activated areas comprised mesial premotor regions corresponding to the location of the supplementary motor area. Bilateral activation also was seen in the superior posterior parietal cortex along the intraparietal sulcus (Fig. 3). Most significantly, however, the hand representation of the primary motor cortex (21) was not activated by the phantom finger-to-thumb opposition tasks. This area also remained silent during pronation-supination of the right phantom hand, which showed a pattern of activation similar to the one detected during phantom finger movements. Phantom hand and finger movements again did not produce any activation in the postcentral gyrus. Flexion-extension of the right phantom foot disclosed activation in premotor and parietal cortical areas and, additionally, a small but statistically significant activation in the contralateral paracentral region (Brodmann area 4).
Figure 3.
Cortical activation areas during self-paced movements of phantom fingers. Two representative sections through the sensorimotor cortical hand areas during fingers-to-thumb opposition with the left (Top) and right (Bottom) phantom fingers. Arrows indicate the anatomical region corresponding to the hand representation in normal subjects (21). The color scale on the left of each section represents Student's t test values [yellow, t = 3.6 (P ≤ 0.001); red, t = 6.9). The anatomical locations of activated areas are listed next to the corresponding sections both as Talairach and Tournoux coordinates and probable Brodmann areas (BA).
fMRI of Existing Body Parts.
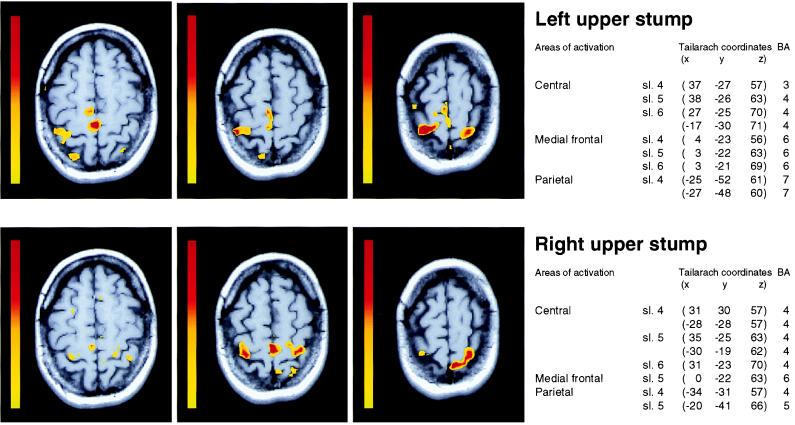
The face muscle contraction tasks activated regions corresponding to their expected somatotopical location; none invaded the silent hand representations. During flexion-extension movements of the upper stumps, we observed an inferior expansion of the proximal arm representations into the hand territories congenitally deprived of afferences and efferences (Fig. 4). Although for the left-sided stump movements, activation of M1 was confined to the right hemisphere, right-sided movements produced a bilateral activation. Similarly, during movements of the right lower stump, significant bilateral activation was elicited in the supposedly silent paracentral areas for foot representation.
Figure 4.
Cortical activation areas during movements of existing body parts. Three contiguous sections covering the sensorimotor cortex from the hand representation up to the vertex during movements of the left (Top) and right (Bottom) upper arm. BA, Brodmann areas. (Color scale and coordinates as in Fig. 3.)
TMS.
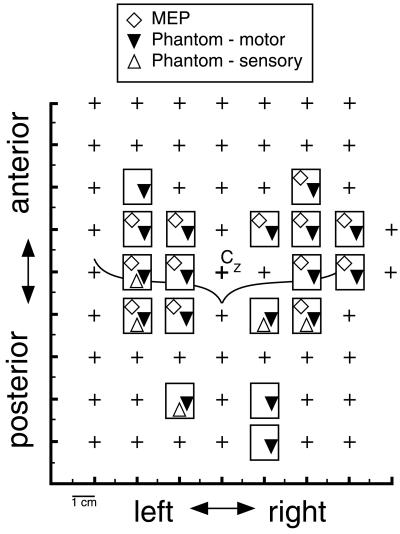
The MEP maps of deltoid and TMS-elicited phantom sensations are displayed in Fig. 5. In 13 of 18 stimulation sites, a coincidence of phantom sensations and deltoid MEPs was found. Most of these sites were located anterior to the central sulcus, thus expanding into the premotor cortex. Centers of gravity of MEP amplitudes for the deltoid muscles were calculated according to ref. 22. They had the following coordinates with reference to Cz: 3.6 cm lateral, 0.5 cm anterior (right hemisphere); and 5.7 cm lateral, 1.5 cm anterior (left hemisphere). TMS-induced phantom movement sensations often followed stimulus application with quite long latencies of more than 1 s. Phantom movements were reported exclusively in the limb contralateral to the hemisphere stimulated and described as slow movements of one or several fingers, of the whole hand, or, more rarely, of the entire forearm. Twitches were never reported in phantom body parts, although they were regularly experienced in the stump. Stimulation at four sites posterior to the central reference mark Cz elicited isolated phantom movement sensations without corresponding MEPs. In two of them, somatosensory phenomena, such as pain and paresthesias, were reported. Pure movement sensations in the phantoms occurred after TMS at one site anterior to M1, probably in the premotor cortex, and in two parietal locations.
Figure 5.
Overlay of MEP map and the subject's reports of phantom hand sensations in response to TMS. Each rectangle represents a stimulation site from which positive responses were obtained. Crosses indicate stimulation sites with neither MEPs nor phantom sensations. The curved line depicts the approximate location of the central sulci.
Discussion
Previous clinical reports on subjects with phantom sensations of congenitally absent limbs have been confined to descriptions of introspective data obtained in patient interviews (2–9). Accordingly, the neural mechanisms proposed to underlie these phantom sensations lacked an empirical foundation. Against this background, we have attempted to set some constraints on a proper theory of aplasic phantoms by applying various experimental methods to a subject with congenital aplasia of all four limbs. The complete absence, in our subject, of both arms well above the elbow joints rules out the possibility that the phantoms of her congenitally absent fingers were mere kinesthetic illusions resulting from abnormally enhanced joint motility of residual phalanges (3). Likewise, a transposition of sensorimotor maps of intact limbs from one hemisphere to the other can be excluded as an explanation of A.Z.'s phantom sensations of both lower and upper limbs (6, 7). The proposal of an innate schema for hand-mouth coordination (9) also does not offer an explanation for our subject's (nor other subjects'; cf. ref. 8) aplasic phantoms of the lower limbs. Finally, if congenital phantom sensations were merely vivid fantasies lacking proper sensory qualities (14), then one would not have expected A.Z. to display differentiated reaction times in the visual-kinesthetic integration task, which implicitly required her to rotate her phantom hand or foot. In subjects with intact limbs, this task activated a widespread system of parietal areas comprising the superior parietal cortex, intraparietal sulcus, and inferior parietal lobule, whereas primary motor and somatosensory cortices were inactive (12, 13). The present fMRI experiments showed a similar pattern of activation (and nonactivation) in our subject while she performed virtual movements with her phantom fingers and hands. Ramachandran et al. (1) speculated that phantom limb sensations may partly originate from the monitoring of efference copy signals or motor commands in the parietal cortex. Our study now provides clear evidence for parietal participation but, in addition, the premotor cortex was consistently activated during phantom movements. Activation was bilateral in both regions, although slightly more pronounced in the hemisphere contralateral to the phantom movement. The striking lack of primary sensorimotor cortex activation during phantom finger movements contrasts with recent fMRI findings in a traumatic amputee (23), as well as our own observations in three similar cases (unpublished data). In all these amputees, repetitive movements of phantom fingers led to an activation of contralateral primary motor areas, which was quantitatively less pronounced than that elicited by movements of the existing fingers. This primary motor activation, also observed in motor imagery studies with healthy volunteers (24), has been interpreted as a confirmation of the theory that phantom movements are sensorimotor memories of lost body parts (23). However, whereas the primary motor areas may indeed participate in mediating intentional phantom movements after longstanding deafferentation, these areas do not seem to contribute to phantom movements of congenitally absent limbs. We interpret the small activation observed in the paracentral areas of foot representation during phantom foot movements as the consequence of involuntary muscle activity in the lower stump (which was not controlled by EMG).
The observation that shoulder movements activated large cortical regions invading parts of the silent hand representations is in accordance with previous positron-emission tomography findings from both traumatic and congenital upper limb amputees (the latter without phantom sensations; ref. 25). However, in that study, the areas were wider in traumatic amputees as compared to congenital amputees, which led Kew et al. (25) to assume a different course of reorganization in subjects with limb aplasia as compared to those who suffered limb deafferentation in later life. Imaging studies are needed that directly compare the brain areas activated by (i) movements of phantom limbs after amputations and (ii) movements of aplasic phantoms. Our observation that face muscle activity produced brain activation exclusively in sensorimotor sites confined to the respective somatotopic locations (without expansion into the neighboring regions of hand representation) is consistent with the behaviorally established absence of referred sensations from the face to upper limb phantoms.
One final comment on the data obtained in the fMRI experiments is germane. The existence in our subject of morphologically normally shaped central sulci with distinct areas of hand representation (ref. 21; Fig. 3) is not self-evident. A 19th-century postmortem examination of the brain of a 40-year-old man born without a left hand revealed an isolated diminution of the right hemisphere ascending parietal convolution (26). Analogous atrophies, both spinal and cortical, frequently were reported in subsequent studies of persons with congenital limb aplasia (see ref. 27 for an early review). These earlier studies, however, did not report whether their subjects had experienced phantom sensations of their missing body parts. Whether the presence or absence of such sensations is causally related to observed alterations in relevant central nervous system structures is a question that has yet to be explored.
TMS revealed a deltoid representation in our subject, unexpectedly large given the relatively low stimulation strength. This finding contradicts a recent study of persons with longstanding traumatic amputations (28) but corresponds to published observations in congenital amputees who did not report phantoms sensations (25, 29). The coordinates of the center of gravity of the deltoid representation were within the 95% confidence interval of control subjects (22). However, because of the bilateral absence of the lower arms in our subject, we lacked an intraindividual reference that would have allowed us to determine conclusively the extent of reorganization. With respect to the mapping of the phantom limbs, we found that the relation between stimulated scalp locations and the reported phantom sensations did not disclose a strictly somatotopic organization with distinct regions for fingers, wrist, and forearm. In contrast to the fMRI finding of a bilateral representation of phantom hand movements, TMS-elicited phantom movement sensations were strictly confined to the side contralateral to stimulation. This result may point to a fundamental difference between externally triggered phantom sensations on the one hand and self-generated phantom movements on the other hand. A notable finding was the quite unanticipated occurrence of phantom limb pain and paresthesias after stimulation at some postrolandic sites. Such uncomfortable sensations had never been experienced spontaneously by the subject. In contrast to previously published observations (30, 31) in subjects with traumatic amputations, our subject did not report any TMS-induced muscle twitches in the phantoms. A further deviation from comparable work with traumatic amputees was the occurrence of phantom movement sensations without concomitant MEPs in our subject (see examples in refs. 30 and 31).
To summarize, our study challenges the view of phantom limbs as a mere product of “re-membering,” in other words, our findings clearly indicate that phantom experiences are not exclusively based on perceptuomotor memories of once-present body parts. Although we can rule out several previously advanced theories of aplasic phantoms, our experimental findings cannot directly address the role of genetic factors in the development of “body schema,” i.e., the central representation of one's own body (32). The existence of aplasic phantoms has often been cited (1, 8, 9, 33) as evidence that this schema (also designated as “neuromatrix”; ref. 33) has an innate component. The present findings neither confirm nor contradict this notion. We prefer to take a more parsimonious stance and stress that both “primordial” and epigenetic factors must be considered. One candidate mediator of aplasic phantom sensations could be the neural networks coding for both action-preparation and action-observation. First described in the monkey premotor cortex (34, 35), neurons that discharge both during the individual's own hand actions and during the observation of another individual performing similar actions have been shown to exist in humans as well (36–39). From a biological perspective, an innate albeit highly plastic schema for matching the observation with the execution of motor actions seems likely. In the absence of a physical substrate for the execution of an action, habitual perception of a conspecies moving extremities could still activate networks mediating a visuomotor limb representation. This activation may give rise to phantom sensations in at least a minority of individuals with limb aplasia. A very early use of action-observation as a basis for action planning is suggested by developmental studies that have shown that infants as young as 6 weeks old are capable of imitating a variety of distinct facial movements (40). On the other hand, gesture usage in congenitally blind people reportedly is comparable to that of sighted people, indicating a genetic component for the execution of communication-related hand and arm movements (41). Although admittedly speculative, the conceptualization of phantom limbs as the phenomenal correlate of planning actions with a nonexistent limb could account for the fact that aplasic phantoms are more frequent for upper as compared to lower limbs (9); clearly, both action-observation and gesturing involve movements of the hands and arms rather than the feet and legs.
Acknowledgments
We thank A.Z. for her participation in this study. H. Schnyder's (Zürich) technical support in the TMS experiment is also acknowledged. For further help and support we thank C. Hess (Bern), T. Landis (Geneva), B. Weber (Zürich), and especially N. D. Cook (Osaka). H. Roth (Zürich) kindly provided the artwork for Fig. 1. This research was supported by the Swiss National Science Foundation Grant 4038-052837.
Abbreviations
- fMRI
functional magnetic resonance imaging
- TMS
transcranial magnetic stimulation
- MEP
motor evoked potentials
- Cz
vertex
- EMG
electromyography
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.100510697.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.100510697
References
- 1.Ramachandran V S, Hirstein W. Brain. 1998;121:1603–1630. doi: 10.1093/brain/121.9.1603. [DOI] [PubMed] [Google Scholar]
- 2.Valentin G. Repertorium Anat Physiol. 1836;1:328–337. [Google Scholar]
- 3.Simmel M L. Am J Psychol. 1961;74:467–470. [Google Scholar]
- 4.Weinstein S, Sersen E A. Neurology. 1961;11:905–911. doi: 10.1212/wnl.11.10.905. [DOI] [PubMed] [Google Scholar]
- 5.Poeck K. Cortex. 1964;1:269–275. [Google Scholar]
- 6.Burchard J M. Arch Psychiatr Z Ges Neurol. 1965;207:360–377. doi: 10.1007/BF00361229. [DOI] [PubMed] [Google Scholar]
- 7.Grouios G. Med Sci Res. 1996;24:503–504. [Google Scholar]
- 8.Melzack R, Israel R, Lacroix R, Schultz G. Brain. 1997;120:1603–1620. doi: 10.1093/brain/120.9.1603. [DOI] [PubMed] [Google Scholar]
- 9.Gallagher S, Butterworth G E, Lew A, Cole J. Brain Cognit. 1998;38:53–65. doi: 10.1006/brcg.1998.1020. [DOI] [PubMed] [Google Scholar]
- 10.Cooper L A, Shepard R N. J Exp Psychol Human Percept Perf. 1975;1:48–56. [PubMed] [Google Scholar]
- 11.Parsons L M. Cognit Psychol. 1987;19:178–241. doi: 10.1016/0010-0285(87)90011-9. [DOI] [PubMed] [Google Scholar]
- 12.Bonda E, Petrides M, Frey S, Evans A. Proc Natl Acad Sci USA. 1995;92:11180–11184. doi: 10.1073/pnas.92.24.11180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parsons L M, Fox P T, Hunter Downs J, Glass T, Hirsch T B, Martin C C, Jerabek P A, Lancaster J L. Nature (London) 1995;375:54–58. doi: 10.1038/375054a0. [DOI] [PubMed] [Google Scholar]
- 14.Skoyles J R. Trends Neurosci. 1990;13:409. doi: 10.1016/0166-2236(90)90120-y. (lett.). [DOI] [PubMed] [Google Scholar]
- 15.Franz E A, Ramachandran V S. Nat Neurosci. 1998;1:443–444. doi: 10.1038/2161. [DOI] [PubMed] [Google Scholar]
- 16.Henderson W R, Smyth G E. J Neurol Neurosurg Psychiatry. 1948;11:88–112. doi: 10.1136/jnnp.11.2.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramachandran V S, Stewart M, Rogers-Ramachandran D C. NeuroReport. 1992;3:583–586. doi: 10.1097/00001756-199207000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Halligan P W, Marshall J C, Wade D T, Davey J, Morrison D. NeuroReport. 1993;4:233–236. doi: 10.1097/00001756-199303000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Ogawa S, Lee T M, Kay A R, Tank D W. Proc Natl Acad Sci USA. 1990;87:8868–8872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woods R P, Grafton S T, Watson J D, Sicotte N L, Mazziotta J C. J Comput Assist Tomogr. 1998;22:153–165. doi: 10.1097/00004728-199801000-00028. [DOI] [PubMed] [Google Scholar]
- 21.Kleinschmidt A, Nitschke M F, Frahm J. Eur J Neurosci. 1997;9:2178–2186. doi: 10.1111/j.1460-9568.1997.tb01384.x. [DOI] [PubMed] [Google Scholar]
- 22.Wassermann E M, McShane L M, Hallett M, Cohen L G. Electroencephalogr Clin Neurophysiol. 1992;85:1–8. doi: 10.1016/0168-5597(92)90094-r. [DOI] [PubMed] [Google Scholar]
- 23.Ersland L, Rosen G, Lundervold A, Smievoll A I, Tillung T, Sundberg H, Hugdahl K. NeuroReport. 1996;8:207–210. doi: 10.1097/00001756-199612200-00042. [DOI] [PubMed] [Google Scholar]
- 24.Schnitzler A, Salenius S, Salmelin R, Jousmäki V, Hari R. Neuroimage. 1997;6:201–208. doi: 10.1006/nimg.1997.0286. [DOI] [PubMed] [Google Scholar]
- 25.Kew J J M, Ridding M C, Rothwell J C, Passingham R E, Leigh P N, Sooriakumaran S, Frackowiak R S J, Brooks D J. J Neurophysiol. 1994;72:2517–2524. doi: 10.1152/jn.1994.72.5.2517. [DOI] [PubMed] [Google Scholar]
- 26.Gowers W R. Brain. 1879;1:386–390. [Google Scholar]
- 27.Edinger L. Arch Pathol Anat Physiol Klin Med. 1882;89:46–63. [Google Scholar]
- 28.Röricht S, Meyer B-U, Niehaus L, Brandt S A. Neurology. 1999;53:106–111. doi: 10.1212/wnl.53.1.106. [DOI] [PubMed] [Google Scholar]
- 29.Hall E J, Flament D, Fraser C, Lemon R N. Neurosci Lett. 1990;116:379–386. doi: 10.1016/0304-3940(90)90105-i. [DOI] [PubMed] [Google Scholar]
- 30.Hess C W, Mills K R, Murray N M F. Neurosci Lett. 1986;71:235–240. doi: 10.1016/0304-3940(86)90565-3. [DOI] [PubMed] [Google Scholar]
- 31.Cohen L G, Bandinelli S, Findley T W, Hallett M. Brain. 1991;114:615–627. doi: 10.1093/brain/114.1.615. [DOI] [PubMed] [Google Scholar]
- 32.Berlucchi G, Aglioti S. Trends Neurosci. 1997;20:560–564. doi: 10.1016/s0166-2236(97)01136-3. [DOI] [PubMed] [Google Scholar]
- 33.Melzack R. Trends Neurosci. 1990;13:88–92. doi: 10.1016/0166-2236(90)90179-e. [DOI] [PubMed] [Google Scholar]
- 34.Di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. Exp Brain Res. 1992;91:176–180. doi: 10.1007/BF00230027. [DOI] [PubMed] [Google Scholar]
- 35.Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Brain. 1996;119:593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- 36.Fadiga L, Fogassi L, Pavesi G, Rizzolatti G. J Neurophysiol. 1995;73:2608–2611. doi: 10.1152/jn.1995.73.6.2608. [DOI] [PubMed] [Google Scholar]
- 37.Grafton S T, Arbib M A, Fadiga L, Rizzolatti G. Exp Brain Res. 1996;112:103–111. doi: 10.1007/BF00227183. [DOI] [PubMed] [Google Scholar]
- 38.Decety J, Grèzes J, Costes N, Perani D, Jeannerod M, Procyk E, Grassi F, Fazio F. Brain. 1997;120:1763–1777. doi: 10.1093/brain/120.10.1763. [DOI] [PubMed] [Google Scholar]
- 39.Hari R, Forss N, Avikainen S, Kirveskari E, Salenius S, Rizzolatti G. Proc Natl Acad Sci USA. 1998;95:15061–15065. doi: 10.1073/pnas.95.25.15061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meltzoff A N, Moore M K. Infant Behav Dev. 1994;17:83–99. doi: 10.1016/0163-6383(94)90024-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iverson J M, Goldin-Meadow S. Nature (London) 1998;396:228. doi: 10.1038/24300. [DOI] [PubMed] [Google Scholar]