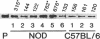Abstract
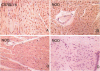
High levels of CD36 expression are found in triglyceride storing and secreting cells such as differentiated adipocytes and mammary secretory epithelial cells and in some capillary endothelial cells. We have found high levels of CD36 in the capillary endothelium of murine adipose tissue and in cardiac and skeletal muscles. Muscle cells themselves were CD36 negative. No CD36 was found in brain endothelium. Cardiac and skeletal muscle tissues are highly oxidative and catabolize long-chain fatty acids as a source of energy while brain tissue does not use long-chain fatty acids for energy production. Since capillary endothelial cell CD36 expression appeared to correlate with parenchymal cell fatty acid utilization and since CD26 has been identified recently as a long-chain fatty acid-binding protein, we examined heart tissue CD36 expression in murine models of insulin-dependent (nonobese diabetic, NOD) and non-insulin-dependent diabetes mellitus (KKAY). Diabetic NOD and KKAY mice had serum triglyceride levels 2.6- and 4.2-fold higher, respectively, than normal mice and exhibited 7- and 3.5-fold higher levels of heart microsomal CD36, respectively, than control mice. Mice fed a 40% fat diet expressed heart tissue CD36 at a level 3.5-fold higher than those fed a 9% fat diet. These data suggest that endothelial cell CD36 expression is related to parenchymal cell lipid metabolism.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abumrad N. A., Melki S. A., Harmon C. M. Transport of fatty acid in the isolated rat adipocyte and in differentiating preadipose cells. Biochem Soc Trans. 1990 Dec;18(6):1130–1132. doi: 10.1042/bst0181130. [DOI] [PubMed] [Google Scholar]
- Abumrad N. A., Park J. H., Park C. R. Permeation of long-chain fatty acid into adipocytes. Kinetics, specificity, and evidence for involvement of a membrane protein. J Biol Chem. 1984 Jul 25;259(14):8945–8953. [PubMed] [Google Scholar]
- Abumrad N. A., Perkins R. C., Park J. H., Park C. R. Mechanism of long chain fatty acid permeation in the isolated adipocyte. J Biol Chem. 1981 Sep 10;256(17):9183–9191. [PubMed] [Google Scholar]
- Abumrad N. A., el-Maghrabi M. R., Amri E. Z., Lopez E., Grimaldi P. A. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J Biol Chem. 1993 Aug 25;268(24):17665–17668. [PubMed] [Google Scholar]
- Acton S. L., Scherer P. E., Lodish H. F., Krieger M. Expression cloning of SR-BI, a CD36-related class B scavenger receptor. J Biol Chem. 1994 Aug 19;269(33):21003–21009. [PubMed] [Google Scholar]
- Akasaki K., Michihara A., Fukuzawa M., Kinoshita H., Tsuji H. Cycling of an 85-kDa lysosomal membrane glycoprotein between the cell surface and lysosomes in cultured rat hepatocytes. J Biochem. 1994 Sep;116(3):670–676. [PubMed] [Google Scholar]
- Argilés J. M., López-Soriano J., López-Soriano F. J. Cytokines and diabetes: the final step? Involvement of TNF-alpha in both type I and II diabetes mellitus. Horm Metab Res. 1994 Oct;26(10):447–449. doi: 10.1055/s-2007-1001730. [DOI] [PubMed] [Google Scholar]
- Asch A. S., Barnwell J., Silverstein R. L., Nachman R. L. Isolation of the thrombospondin membrane receptor. J Clin Invest. 1987 Apr;79(4):1054–1061. doi: 10.1172/JCI112918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt R., Pepperle M., Otto A., Kraft R., Boehmer F. D., Grosse R. A 13-kilodalton protein purified from milk fat globule membranes is closely related to a mammary-derived growth inhibitor. Biochemistry. 1988 Mar 8;27(5):1420–1425. doi: 10.1021/bi00405a005. [DOI] [PubMed] [Google Scholar]
- Burton P. B., Hogben C. E., Joannou C. L., Clark A. G., Hsuan J. J., Totty N. F., Sorensen C., Evans R. W., Tynan M. J. Heart fatty acid binding protein is a novel regulator of cardiac myocyte hypertrophy. Biochem Biophys Res Commun. 1994 Dec 30;205(3):1822–1828. doi: 10.1006/bbrc.1994.2882. [DOI] [PubMed] [Google Scholar]
- Calvo D., Vega M. A. Identification, primary structure, and distribution of CLA-1, a novel member of the CD36/LIMPII gene family. J Biol Chem. 1993 Sep 5;268(25):18929–18935. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Endemann G., Stanton L. W., Madden K. S., Bryant C. M., White R. T., Protter A. A. CD36 is a receptor for oxidized low density lipoprotein. J Biol Chem. 1993 Jun 5;268(16):11811–11816. [PubMed] [Google Scholar]
- Fujita T., Sugiyama Y., Taketomi S., Sohda T., Kawamatsu Y., Iwatsuka H., Suzuoki Z. Reduction of insulin resistance in obese and/or diabetic animals by 5-[4-(1-methylcyclohexylmethoxy)benzyl]-thiazolidine-2,4-dione (ADD-3878, U-63,287, ciglitazone), a new antidiabetic agent. Diabetes. 1983 Sep;32(9):804–810. doi: 10.2337/diab.32.9.804. [DOI] [PubMed] [Google Scholar]
- Glatz J. F., van Breda E., Keizer H. A., de Jong Y. F., Lakey J. R., Rajotte R. V., Thompson A., van der Vusse G. J., Lopaschuk G. D. Rat heart fatty acid-binding protein content is increased in experimental diabetes. Biochem Biophys Res Commun. 1994 Mar 15;199(2):639–646. doi: 10.1006/bbrc.1994.1276. [DOI] [PubMed] [Google Scholar]
- Greenwalt D. E., Lipsky R. H., Ockenhouse C. F., Ikeda H., Tandon N. N., Jamieson G. A. Membrane glycoprotein CD36: a review of its roles in adherence, signal transduction, and transfusion medicine. Blood. 1992 Sep 1;80(5):1105–1115. [PubMed] [Google Scholar]
- Greenwalt D. E., Mather I. H. Characterization of an apically derived epithelial membrane glycoprotein from bovine milk, which is expressed in capillary endothelia in diverse tissues. J Cell Biol. 1985 Feb;100(2):397–408. doi: 10.1083/jcb.100.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwalt D. E., Watt K. W., So O. Y., Jiwani N. PAS IV, an integral membrane protein of mammary epithelial cells, is related to platelet and endothelial cell CD36 (GP IV). Biochemistry. 1990 Jul 31;29(30):7054–7059. doi: 10.1021/bi00482a015. [DOI] [PubMed] [Google Scholar]
- Harmon C. M., Abumrad N. A. Binding of sulfosuccinimidyl fatty acids to adipocyte membrane proteins: isolation and amino-terminal sequence of an 88-kD protein implicated in transport of long-chain fatty acids. J Membr Biol. 1993 Apr;133(1):43–49. doi: 10.1007/BF00231876. [DOI] [PubMed] [Google Scholar]
- Harmon C. M., Luce P., Beth A. H., Abumrad N. A. Labeling of adipocyte membranes by sulfo-N-succinimidyl derivatives of long-chain fatty acids: inhibition of fatty acid transport. J Membr Biol. 1991 May;121(3):261–268. doi: 10.1007/BF01951559. [DOI] [PubMed] [Google Scholar]
- Hart K., Wilcox M. A Drosophila gene encoding an epithelial membrane protein with homology to CD36/LIMP II. J Mol Biol. 1993 Nov 5;234(1):249–253. doi: 10.1006/jmbi.1993.1580. [DOI] [PubMed] [Google Scholar]
- Huang M. M., Bolen J. B., Barnwell J. W., Shattil S. J., Brugge J. S. Membrane glycoprotein IV (CD36) is physically associated with the Fyn, Lyn, and Yes protein-tyrosine kinases in human platelets. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7844–7848. doi: 10.1073/pnas.88.17.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh H. Y., Lo S. K., Yesner L. M., Silverstein R. L. CD36 induction on human monocytes upon adhesion to tumor necrosis factor-activated endothelial cells. J Biol Chem. 1995 Mar 17;270(11):6267–6271. doi: 10.1074/jbc.270.11.6267. [DOI] [PubMed] [Google Scholar]
- Iwatsuka H., Shino A., Suzuoki Z. General survey of diabetic features of yellow KK mice. Endocrinol Jpn. 1970 Feb;17(1):23–35. doi: 10.1507/endocrj1954.17.23. [DOI] [PubMed] [Google Scholar]
- Jochen A., Hays J. Purification of the major substrate for palmitoylation in rat adipocytes: N-terminal homology with CD36 and evidence for cell surface acylation. J Lipid Res. 1993 Oct;34(10):1783–1792. [PubMed] [Google Scholar]
- Kamp F., Hamilton J. A., Kamp F., Westerhoff H. V., Hamilton J. A. Movement of fatty acids, fatty acid analogues, and bile acids across phospholipid bilayers. Biochemistry. 1993 Oct 19;32(41):11074–11086. doi: 10.1021/bi00092a017. [DOI] [PubMed] [Google Scholar]
- Knowles D. M., 2nd, Tolidjian B., Marboe C., D'Agati V., Grimes M., Chess L. Monoclonal anti-human monocyte antibodies OKM1 and OKM5 possess distinctive tissue distributions including differential reactivity with vascular endothelium. J Immunol. 1984 May;132(5):2170–2173. [PubMed] [Google Scholar]
- Makino S., Kunimoto K., Muraoka Y., Mizushima Y., Katagiri K., Tochino Y. Breeding of a non-obese, diabetic strain of mice. Jikken Dobutsu. 1980 Jan;29(1):1–13. doi: 10.1538/expanim1978.29.1_1. [DOI] [PubMed] [Google Scholar]
- Neely J. R., Rovetto M. J., Oram J. F. Myocardial utilization of carbohydrate and lipids. Prog Cardiovasc Dis. 1972 Nov-Dec;15(3):289–329. doi: 10.1016/0033-0620(72)90029-1. [DOI] [PubMed] [Google Scholar]
- Nicholson A. C., Frieda S., Pearce A., Silverstein R. L. Oxidized LDL binds to CD36 on human monocyte-derived macrophages and transfected cell lines. Evidence implicating the lipid moiety of the lipoprotein as the binding site. Arterioscler Thromb Vasc Biol. 1995 Feb;15(2):269–275. doi: 10.1161/01.atv.15.2.269. [DOI] [PubMed] [Google Scholar]
- Ren Y., Silverstein R. L., Allen J., Savill J. CD36 gene transfer confers capacity for phagocytosis of cells undergoing apoptosis. J Exp Med. 1995 May 1;181(5):1857–1862. doi: 10.1084/jem.181.5.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer J. E., Lodish H. F. Expression cloning and characterization of a novel adipocyte long chain fatty acid transport protein. Cell. 1994 Nov 4;79(3):427–436. doi: 10.1016/0092-8674(94)90252-6. [DOI] [PubMed] [Google Scholar]
- Schwieterman W., Sorrentino D., Potter B. J., Rand J., Kiang C. L., Stump D., Berk P. D. Uptake of oleate by isolated rat adipocytes is mediated by a 40-kDa plasma membrane fatty acid binding protein closely related to that in liver and gut. Proc Natl Acad Sci U S A. 1988 Jan;85(2):359–363. doi: 10.1073/pnas.85.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüepp B. J., Pfister H., Clemetson K. J., Silverstein R. L., Jungi T. W. CD36-mediated signal transduction in human monocytes by anti-CD36 antibodies but not by anti-thrombospondin antibodies recognizing cell membrane-bound thrombospondin. Biochem Biophys Res Commun. 1991 Feb 28;175(1):263–270. doi: 10.1016/s0006-291x(05)81229-x. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Storch J., Lechene C., Kleinfeld A. M. Direct determination of free fatty acid transport across the adipocyte plasma membrane using quantitative fluorescence microscopy. J Biol Chem. 1991 Jul 25;266(21):13473–13476. [PubMed] [Google Scholar]
- Stremmel W., Lotz G., Strohmeyer G., Berk P. D. Identification, isolation, and partial characterization of a fatty acid binding protein from rat jejunal microvillous membranes. J Clin Invest. 1985 Mar;75(3):1068–1076. doi: 10.1172/JCI111769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon N. N., Kralisz U., Jamieson G. A. Identification of glycoprotein IV (CD36) as a primary receptor for platelet-collagen adhesion. J Biol Chem. 1989 May 5;264(13):7576–7583. [PubMed] [Google Scholar]
- Tandon N. N., Lipsky R. H., Burgess W. H., Jamieson G. A. Isolation and characterization of platelet glycoprotein IV (CD36). J Biol Chem. 1989 May 5;264(13):7570–7575. [PubMed] [Google Scholar]
- Tochino Y. The NOD mouse as a model of type I diabetes. Crit Rev Immunol. 1987;8(1):49–81. [PubMed] [Google Scholar]
- Trezzini C., Jungi T. W., Spycher M. O., Maly F. E., Rao P. Human monocytes CD36 and CD16 are signaling molecules. Evidence from studies using antibody-induced chemiluminescence as a tool to probe signal transduction. Immunology. 1990 Sep;71(1):29–37. [PMC free article] [PubMed] [Google Scholar]
- Turner G. D., Morrison H., Jones M., Davis T. M., Looareesuwan S., Buley I. D., Gatter K. C., Newbold C. I., Pukritayakamee S., Nagachinta B. An immunohistochemical study of the pathology of fatal malaria. Evidence for widespread endothelial activation and a potential role for intercellular adhesion molecule-1 in cerebral sequestration. Am J Pathol. 1994 Nov;145(5):1057–1069. [PMC free article] [PubMed] [Google Scholar]
- Van Nieuwenhoven F. A., Verstijnen C. P., Abumrad N. A., Willemsen P. H., Van Eys G. J., Van der Vusse G. J., Glatz J. F. Putative membrane fatty acid translocase and cytoplasmic fatty acid-binding protein are co-expressed in rat heart and skeletal muscles. Biochem Biophys Res Commun. 1995 Feb 15;207(2):747–752. doi: 10.1006/bbrc.1995.1250. [DOI] [PubMed] [Google Scholar]
- Vega M. A., Seguí-Real B., García J. A., Calés C., Rodríguez F., Vanderkerckhove J., Sandoval I. V. Cloning, sequencing, and expression of a cDNA encoding rat LIMP II, a novel 74-kDa lysosomal membrane protein related to the surface adhesion protein CD36. J Biol Chem. 1991 Sep 5;266(25):16818–16824. [PubMed] [Google Scholar]
- Waterston R., Martin C., Craxton M., Huynh C., Coulson A., Hillier L., Durbin R., Green P., Shownkeen R., Halloran N. A survey of expressed genes in Caenorhabditis elegans. Nat Genet. 1992 May;1(2):114–123. doi: 10.1038/ng0592-114. [DOI] [PubMed] [Google Scholar]
- Witztum J. L., Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest. 1991 Dec;88(6):1785–1792. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Spitzer E., Kenney N., Zschiesche W., Li M., Kromminga A., Müller T., Spener F., Lezius A., Veerkamp J. H. Members of the fatty acid binding protein family are differentiation factors for the mammary gland. J Cell Biol. 1994 Nov;127(4):1097–1109. doi: 10.1083/jcb.127.4.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]