Abstract
Aim
To assess the efficacy and safety of an intravitreal injection of bevacizumab (Avastin®) for myopic choroidal neovascularisation (mCNV).
Methods
Intravitreal bevacizumab (1 mg) was injected into eight eyes of eight patients with mCNV in this non‐randomised, interventional case series. The best‐corrected visual acuity (BCVA) was measured and the optical coherence tomography (OCT) and fluorescein angiography findings were examined before and after treatment. The minimum follow‐up time was 3 months.
Results
The mean BCVA was 0.26 before treatment and 0.51 at the last visit (p = 0.009). The BCVA improved to two or more lines in six eyes (75%) and remained the same in two eyes (25%). Leakage from the mCNV on fluorescein angiography decreased in seven eyes (87.5%). The choroidal neovascularisation area on fluorescein angiography (p = 0.049) and the foveal thickness on OCT images decreased significantly (p = 0.027) after the treatment. No major complications developed.
Conclusion
Intravitreal injection of bevacizumab seems to be an effective and safe treatment for mCNV.
Choroidal neovascularisation (CNV) secondary to pathological myopia (mCNV) causes severe visual loss for young and middle‐aged patients, especially in Asia and Europe.1 Once mCNV develops, its prognosis is poor.2 Although several treatments including thermal laser photocoagulation,3,4 photodynamic therapy (PDT),5,6,7,8 macular translocation,9 and surgical removal of mCNV10 have been attempted to treat this disease, the beneficial effects are questionable because of the severe complications, poor long‐term results, or both.
Vascular endothelial growth factor (VEGF) is believed to be a key factor in the development and progression of CNV,11,12 and anti‐VEGF treatments are expected to overcome the disadvantages of conventional treatments. Thus far, several anti‐VEGF treatments have been used to treat CNV owing to age‐related macular degeneration (AMD) and have achieved favourable results.13 Pegaptanib (Macugen®, Eyetech Pharmaceuticals, New York, New York, USA) is an aptamer that targets VEGF 16514 and ranibizumab (Lucentis®, Novartis Pharmaceuticals Corporation, East Hanover, New Jersey, USA) is an antibody fragment targeting all VEGF isoforms.15,16 Bevacizumab (Avastin®, Genentech, South San Francisco, California, USA), also an anti‐VEGF drug originally developed to treat metastatic carcinoma of the colon and rectum,17 is a recombinant humanised monoclonal antibody against all VEGF isoforms. CNV secondary to AMD was recently reported to regress after intravenous or intravitreal injection of bevacizumab.18,19,20,21
The efficiency of intravenous injection of bevacizumab to treat mCNV has been reported in two patients.22 However, systemic administration of bevacizumab can induce adverse events such as cerebral and myocardial infarction. Local application, including intravitreal injection, can avoid such complications. The purpose of this study was to determine the efficacy and safety of intravitreal injection of bevacizumab for mCNV.
Patients and methods
Patient selection
Eight eyes of eight patients with mCNV who presented to the Osaka University Hospital, Osaka, Japan, were included in this prospective interventional case study. Inclusion criteria were pathological myopia, defined as a spherical equivalent less than−8.0 D23; patient age >30 years; baseline BCVA worse than 0.7 (20/30); active subfoveal or juxtafoveal CNV confirmed with fluorescein angiography; and the absence of other ocular diseases that could affect the BCVA. Informed consent was obtained from all patients. The institutional review board approved the study.
Examination
Patient's age, sex, affected eye, spherical equivalent refraction, preoperative duration of symptoms and preoperative treatment were recorded before treatment; the BCVA was recorded before and after treatment. The patients were followed every month with a routine eye examination including a fundus check with dilated pupils, optical coherence tomography (OCT), and measurement of the BCVA using a Landolt C chart by a masked examiner.
Measurement of foveal thickness and CNV
The retinal architecture was evaluated using the OCT 3000 (Zeiss Humphrey Instruments, Dublin, California, USA) with the cross‐hair mode default setting (5.65 mm). The foveal thickness on the OCT image was also measured. A decrease of >10% from the baseline thickness was defined as a reduction and an increase of >10% as an increase compared with the foveal thickness before treatment. The activity of mCNV was evaluated in the late phase (mean (standard deviation (SD)) time 10 (2) min) of fluorescein angiography, carried out before and more than 2 months after treatment.
The fluorescein angiogram photograph was digitalised using ImageNet® (Topcon, Tokyo, Japan), and the CNV was measured on the computer. Both CNV and disc size were measured during the early phase (30 (5) s) by ImageNet® and the CNV area was divided by the disc area. The CNV size is presented as disc areas (DA). A reduction of >10% from the baseline was defined as a reduction and an increase of >10% was defined as an increase compared with the baseline size. The leakage from the CNV was examined in the late phase (10–12 min) compared with the early phase (1–2 min). The leakage was compared between the times before and after treatment, and is described as resolved, reduced, unchanged or increased.
Procedure
After topical anaesthesia, the eye and lids were disinfected with povidone iodine. One milligram of bevacizumab was injected 3.5–4.0 mm posterior to the corneal limbus into the vitreous cavity using a 29‐gauge needle. The injection site was compressed for a minute with a cotton swab to avoid reflux when removing the needle.
Statistical analysis
The effect of bevacizumab was evaluated by changes in the BCVA, foveal thickness and CNV size measured by fluorescein angiography after treatment. Data were analysed using the paired t test or Mann–Whitney U test. The BCVA levels were converted to logMAR equivalents and analysed. A p value <0.05 was considered significant.
Results
Table 1 shows the patient's characteristics. Eight patients (3 men and 5 women) ranged in age from 35 to 70 years (50.5 (11.1) years). The duration of symptoms before treatment ranged from 1 to 30 months (10.6 (9.8) months), the spherical equivalent refraction from −22.0 to −10.5 D (−15.5 (3.8) D), and the follow‐up period after treatment from 3 to 7 months (4.4 (1.4) months). Patients 3 and 6 had received sub‐Tenon's injections of triamcinolone before treatment (patient 3, 1 month before treatment; patient 6 two injections, 17 and 26 months before treatment). The vision worsened and the CNV enlarged after the sub‐Tenon's injection in patient 3, and the patient switched treatment. No major complications, including infectious endophthalmitis, vitreous haemorrhage, or retinal detachment, developed during the follow‐up period. A second injection was given in patient 1 (2 months after the initial injection) and in patients 2 and 3 (1 month after injection) because the mCNV was still active.
Table 1 Demographic data of patients with mCNV.
| Patient follow‐up No/sex/age (years) | Duration (Months) | R/L | Refraction (D) | Position of (CNV) | BCVA | Foveal thickness (µm) | CNV size (DA) | Change of | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Final | Pre | Final | Pre | Final | Fluorescein leakage | Month | |||||
| 1/M/38 | 6 | L | −14.75 | Subfoveal | 0.09 | 0.5* | 166 | 150 | 2.07 | 1.98 | Reduced | 7 |
| 2/M/49 | 19 | L | −18.00 | Subfoveal | 0.3 | 0.6* | 245 | 147* | 0.51 | 0.43* | Reduced | 6 |
| 3/F/57 | 1 | R | −22.00 | Juxtafoveal | 0.1 | 0.5* | 202 | 119* | 0.07 | 0.03* | Resolved | 4 |
| 4/F/70 | 13 | R | −11.00† | Subfoveal | 0.3 | 0.6* | 158 | 87* | 0.12 | 0.10* | Reduced | 3.5 |
| 5/M/50 | 4 | R | −14.50 | Subfoveal | 0.5 | 0.8* | 186 | 107* | 0.12 | 0.07* | Resolved | 3 |
| 6/F/35 | 30 | L | −10.50 | Subfoveal | 0.7 | 0.8 | 87 | 94 | 0.19 | 0.18 | Unchanged | 3.5 |
| 7/F/48 | 9 | R | −17.50 | Subfoveal | 0.4 | 0.4 | 309 | 293 | 1.67 | 1.38* | Reduced | 4 |
| 8/F/57 | 3 | R | −15.50 | Juxtafoveal | 0.2 | 0.35* | 234 | 244 | 0.97 | 0.94 | Reduced | 4 |
D, diopters; Duration, duration of symptoms before treatment; F, female; M, male; R, right; L, left; Refraction, spherical equivalent refraction; Pre, pretreatment.
*BCVA improved to more than two lines, foveal thickness and CNV size decreased by more than 10%.
†The refraction before cataract surgery.
The BCVA levels before treatment ranged from 0.09 to 0.7 (mean 0.26). The BCVA at the final visit ranged from 0.35 to 0.8 (mean 0.51). Six patients (75%) had an improved BCVA of two or more lines; in two (25%) patients the BCVA remained the same at the last visit (table 1, p = 0.009 by paired t test). The CNV apparently regressed in some patients after treatment (fig 1A,B). However, the change in the CNV size was unclear on fundus photographs in other cases (figs 2A,B and 3A,B). The fluorescein angiogram showed no angiographic leakage in two of eight eyes (25%); fig 1C,D), reduced leakage in five eyes (62.5%; fig 2C,D), and no change in one eye (12.5%; fig 3C,D). No eyes had an increased angiographic leakage after treatment.
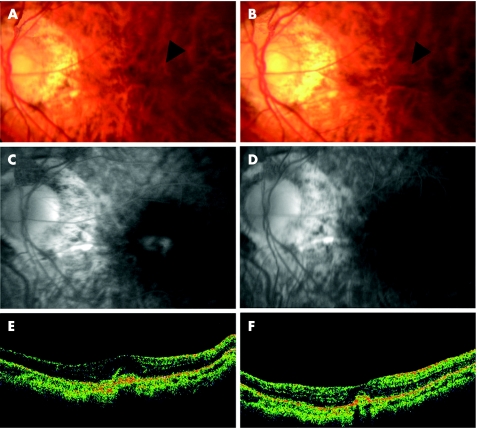
Figure 1 Case 5. (A) Fundus photograph showing the myopic choroidal neovascularisation (mCNV) before treatment (arrowhead). (B) Fundus photograph showing a smaller area of choroidal neovascularisation after treatment (arrowhead). (C) The fluorescein angiography (FA) in the late phase (10 min) showing leakage from the mCNV (arrowhead) before treatment. (D) FA in the late phase (11 min) showing no leakage (arrowhead) from the mCNV 3 months after injection. (E) Vertical optical coherence tomography (OCT) image before treatment showing raised retinal reflectivity and a hyper‐reflective lesion under the retina that corresponds to the mCNV. The thickness of the fovea is 186 µm. (F) OCT image 3 months after intravitreal injection showing a smaller high‐density lesion under the retinal reflectivity compared with before treatment. The thickness of the foveal retina is 107 µm.
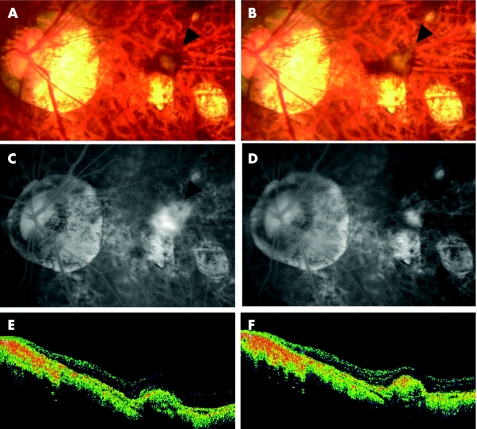
Figure 2 Case 2. (A) Fundus photograph showing the myopic choroidal neovascularisation (mCNV) before treatment (arrowhead). (B) Fundus photograph showing choroidal neovascularisation after treatment (arrowhead). (C) Fluorescein angiography (FA) in the late phase (10 min) showing leakage from the mCNV (arrowhead) before treatment. (D) FA in the late phase (12 min) showing reduced leakage from the mCNV (arrowhead) 4 months after injection. (E) Horizontal optical coherence tomography (OCT) image showing raised retinal reflectivity and a hyper‐reflective lesion under the retina that corresponds to the mCNV before treatment. The thickness of the swollen fovea is 245 µm. (F) OCT image 5 months after intravitreal injection showing the smaller high‐density lesion under the retinal reflectivity compared with that before treatment. The thickness of the foveal retina decreased to 147 µm. The BCVA improved from 0.3 to 0.6.
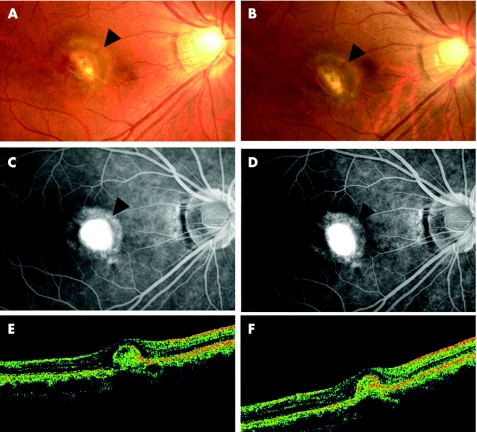
Figure 3 Case 6. (A) Fundus photograph showing the myopic choroidal neovascularisation (mCNV) before treatment (arrowhead). (B) Fundus photograph showing choroidal neovascularisation after treatment (arrowhead). (C) Fluorescein angiography (FA) in the late phase (10 min) showing staining and little leakage from the mCNV (arrowhead) before injection. (D) FA in the late phase (10 min) showing almost the same staining and leakage from the mCNV (arrowhead) 2 months after injection compared with that before treatment. (E) Vertical optical coherence tomography (OCT) image showing raised retinal reflectivity and a hyperreflective round lesion under the retina corresponding to the mCNV before treatment. The thickness of the fovea is 87 µm. (F) OCT image 3.5 months after intravitreal injection showing high‐density lesion of the same size under the retinal reflectivity compared with that before treatment. The thickness of the foveal retina is 94 µm.
The mean (SD) CNV size was 0.72 (0.78) Da before treatment, which decreased significantly to 0.64 (0.73) DA after treatment (p = 0.049 by paired t test, table 1). The CNV size decreased after treatment in 5 (62.5%) eyes and was unchanged in 3 (37.5%). The CNV area decreased in 4 (80%) of 5 eyes with CNV <0.5 Da. However, it decreased in 1 (33.3%) of 3 eyes with a larger area of CNV.
Table 1 shows the foveal thickness of each patient. The mean (SD) foveal thickness was 198.4 (66.5) µm before treatment and 155.1(74.6 µm) after treatment, a difference that reached significance (p = 0.027 by paired t test). The retinal thickness decreased in 4 of 8 (50%) eyes (fig 1E,F and fig 2E,F) and remained the same in 4 (50%) (fig 3E,F).
Discussion
Several studies have reported the efficacy of intravitreal injection of bevacizumab in treating CNV associated with AMD. Avery et al20 reported that most patients had reduced angiographic leakage from neovascular AMD and the BCVA improved noticeably. Other investigators reported similar results.21 The current study showed reduced angiographic leakage from mCNV in 87.5% of eyes, a noticeable improvement in BCVA, a noticeable reduction in foveal thickness, and a noticeable reduction in CNV size after intravitreal bevacizumab injection. Two eyes had increased visual acuity but no reduction in CNV size or foveal thickness (patients 1 and 8). Reduction of foveal leakage on fluorescein angiography may explain the visual improvement after bevacizumab injection. Yoshida et al2 reported that 21% of eyes with mCNV improved to more than one to three lines of BCVA over 3 months during the natural course of the disease. It was also reported that the BCVA improved to more than one to three lines in 25% of eyes with mCNV 3 months after PDT.5 This study showed that the BCVA improved to two or more lines in 75% (six of eight eyes). Although they are not totally comparable, our results seem to be favourable at this time point. A recent experimental study reported that bevacizumab may penetrate the full retinal thickness.24 This finding is consistent with our results showing the effect of bevacizumab on subretinal CNV.
General administration of bevacizumab also seems to be an efficient treatment for mCNV, although only two case reports were presented.22 However, general side effects are a major concern with systemic administration. No major ocular side effects were observed during the follow‐up period in this study. We used 1 mg intravitreal bevacizumab, because that dose was used in the first study of intravitreal bevacizumab to treat eyes with AMD.19 Others showed that 1.25 mg injected intravitreally is well tolerated.20,21 Recent experimental data showed that up to 2.5 mg bevacizumab did not appear toxic to the retina in rabbits.24,25 The vitreous cavity in highly myopic eyes is generally larger because of the elongated axial length, which can dilute the concentration of bevacizumab. However, the turnover of intravitreal bevacizumab may be slower because the function of the thinner retina and the retinal pigment epithelium in highly myopic eyes may deteriorate. Thus, long‐term side effects after injection of 1 mg bevacizumab intravitreally should be investigated in a future study.
CNV owing to AMD has been well investigated, and VEGF has been confirmed as contributing to development and progression of CNV.12 However, the specific pathogenesis in patients with myopia has been poorly described. The administration of bevacizumab led to contraction of mCNV in most of our patients. Successful results in this study indicate that VEGF has an important role at least in the growth of mCNV.
This is the first report of intravitreal injections of bevacizumab into pathological myopic eyes with mCNV. Owing to the nature of this study design and the short‐term follow‐up, the substantial beneficial effects of bevacizumab on mCNV are still uncertain. However, our study results disclosed that bevacizumab might have possible beneficial effects for mCNV. The standard options for CNV, such as PDT, Macugen, or Lucentis, have not been officially approved in many countries. Thus, alternative treatments are needed. While a randomised controlled trial with a larger number of patients with mCNV is needed to prove the efficiency, safety and effective duration of this treatment, our preliminary data suggest that the intravitreal injection of bevacizumab seems to be useful for at least some patients with this disease.
Abbreviations
AMD - age‐related macular degeneration
BCVA - best‐corrected visual acuity
CNV - choroidal neovascularisation
mCNV - myopic choroidal neovascularisation
OCT - optical coherence tomography
PDT - photodynamic therapy
VEGF - vascular endothelial growth factor
Footnotes
This work was Supported by Health Sciences Research Grant from the Ministry of Health, Labor and Welfare, Japan. We have no proprietary interest in any aspect of this report.
Competing interests: None declared.
References
- 1.Tano Y. Pathologic myopia: where are we now? Am J Ophthalmol 2002134645–660. [DOI] [PubMed] [Google Scholar]
- 2.Yoshida T, Ohno‐Matsui K, Yasuzumi K.et al Myopic choroidal neovascularization: a 10‐year follow‐up. Ophthalmology 20031101297–1305. [DOI] [PubMed] [Google Scholar]
- 3.Secretan M, Kuhn D, Soubrane G.et al Long‐term visual outcome of choroidal neovascularization in pathologic myopia: natural history and laser treatment. Eur J Ophthalmol 19977307–316. [DOI] [PubMed] [Google Scholar]
- 4.Johnson D A, Yannuzzi L A, Shakin J L.et al Lacquer cracks following laser treatment of choroidal neovascularization in pathologic myopia. Retina 199818118–124. [DOI] [PubMed] [Google Scholar]
- 5.Verteporfin in Photodynamic Therapy Study Group Photodynamic therapy of subfoveal choroidal neovascularization in pathologic myopia with verteporfin. 1‐year results of a randomized clinical trial‐‐VIP report no. 1. Ophthalmology 2001108841–852. [DOI] [PubMed] [Google Scholar]
- 6.Verteporfin in Photodynamic Therapy (VIP) Study Group Verteporfin therapy of subfoveal choroidal neovascularization in pathologic myopia. 2‐year results of a randomized clinical trial–VIP report no. 3. Ophthalmology 2003110667–673. [DOI] [PubMed] [Google Scholar]
- 7.Ohno‐Matsui K, Moriyama M, Hayashi K.et al Choroidal vein and artery occlusion following photodynamic therapy in eyes with pathologic myopia. Graefe's Arch Clin Exp Ophthalmol 20062441363–1366. [DOI] [PubMed] [Google Scholar]
- 8.Krebs I, Binder S, Stolba U.et al Choroidal neovascularization in pathologic myopia: three‐year results after photodynamic therapy. Am J Ophthalmol 2005140416–425. [DOI] [PubMed] [Google Scholar]
- 9.Ohji M, Fujikado T, Kusaka S.et al Comparison of three techniques of foveal translocation in patients with subfoveal choroidal neovascularization resulting from age‐related macular degeneration. Am J Ophthalmol 2001132888–896. [DOI] [PubMed] [Google Scholar]
- 10.Ruiz‐Moreno J M, de la Vega C. Surgical removal of subfoveal choroidal neovascularisation in highly myopic patients. Br J Ophthalmol 2001851041–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishibashi T, Hata Y, Yoshikawa H.et al Expression of vascular endothelial growth factor in experimental choroidal neovascularization. Graefe's Arch Clin Exp Ophthalmol 1997235159–167. [DOI] [PubMed] [Google Scholar]
- 12.Kwak N, Okamoto N, Wood J M.et al VEGF is major stimulator in model of choroidal neovascularization. Invest Ophthalmol Vis Sci 2000413158–3164. [PubMed] [Google Scholar]
- 13.Gragoudas E S, Adamis A P, Cunningham E T., Jret al Pegaptanib for neovascular age‐related macular degeneration. N Engl J Med 20043512805–2816. [DOI] [PubMed] [Google Scholar]
- 14.Eyetech Study Group Anti‐vascular endothelial growth factor therapy for subfoveal choroidal neovascularization secondary to age‐related macular degeneration: phase II study results. Ophthalmology 2003110979–986. [DOI] [PubMed] [Google Scholar]
- 15.Mordenti J, Cuthbertson R A, Ferrara N.et al Comparisons of the intraocular tissue distribution, pharmacokinetics, and safety of 125I‐labeled full‐length and Fab antibodies in rhesus monkeys following intravitreal administration. Toxicol Pathol 199927536–544. [DOI] [PubMed] [Google Scholar]
- 16.Rosenfeld P J, Heier J S, Hantsbarger G.et al Tolerability and efficacy of multiple escalating doses of ranibizumab (Lucentis) for neovascular age‐related macular degeneration. Ophthalmology 2006113632. [DOI] [PubMed] [Google Scholar]
- 17.Hurwitz H, Fehrenbacher L, Novotny W.et al Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 20043502335–2342. [DOI] [PubMed] [Google Scholar]
- 18.Michels S, Rosenfeld P J, Puliafito C A.et al Systemic bevacizumab (Avastin) therapy for neovascular age‐related macular degeneration twelve‐week results of an uncontrolled open‐label clinical study. Ophthalmology 20051121035–1047. [DOI] [PubMed] [Google Scholar]
- 19.Rosenfeld P J, Moshfeghi A A, Puliafito C A. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for neovascular age‐related macular degeneration. Ophthalmic Surg Lasers Imaging 200536331–335. [PubMed] [Google Scholar]
- 20.Avery R L, Pieramici D J, Rabena M D.et al Intravitreal bevacizumab (Avastin) for neovascular age‐related macular degeneration. Ophthalmology 2006113363–372. [DOI] [PubMed] [Google Scholar]
- 21.Spaide R F, Laud K, Fine H F.et al Intravitreal bevacizumab treatment of choroidal neovascularization secondary to age‐related macular degeneration. Retina 200626383–390. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen Q D, Shah S, Tatlipinar S.et al Bevacizumab suppresses choroidal neovascularisation caused by pathological myopia. Br J Ophthalmol 2005891368–1370. [PMC free article] [PubMed] [Google Scholar]
- 23.Tokoro T. On the definition of pathologic myopia in group studies. Acta Ophthalmol 1988185107–108. [DOI] [PubMed] [Google Scholar]
- 24.Shahar J, Avery R L, Heilweil G.et al Electrophysiologic and retinal penetration studies following intravitreal injection of bevacizumab (Avastin). Retina 200626262–269. [DOI] [PubMed] [Google Scholar]
- 25.Manzano R P, Peyman G A, Khan P.et al Testing intravitreal toxicity of bevacizumab (Avastin). Retina 200626257–261. [DOI] [PubMed] [Google Scholar]