Abstract
Aims
To characterise the distribution of silicone oil in ocular tissues in globes enucleated after complicated retinal detachment, and to document the distribution and nature of any associated inflammatory response.
Method
9 enucleated globes that had previously undergone retinal detachment surgery with silicone oil and 7 control globes that had undergone enucleation after retinal detachment surgery (n = 2) or ocular trauma (n = 5) were studied. Sections were histologically examined using light microscopy to document the distribution of silicone oil in ocular tissues. Immunohistochemical analysis was carried out using the ABC technique and a panel of monoclonal and polyclonal antibodies. Electron microscopy was undertaken to observe the penetration of silicone oil in the trabecular meshwork of the anterior chamber drainage angle.
Results
Silicone oil was distributed throughout the globes—notably in the iris, ciliary body, retina, trabecular meshwork and epiretinal membranes. Focal areas of intraretinal silicone were associated with disorganised retinal architecture, retinectomy sites or subretinal oil. The distribution of macrophages was closely related to the distribution of silicone oil. T and B lymphocytes were not associated with silicone oil unless additional pathology was also present—for example, cyclitic membrane or uveitis. One of the nine eyes had silicone oil present in the optic nerve. In the control globes, the inflammatory response was mediated primarily by macrophages and T lymphocytes, and was less marked than that observed in the silicone oil globes.
Conclusion
This study shows that silicone oil may be sequestered in varied ocular tissues and is associated with localised inflammation mediated by macrophages.
Silicone oil is often used as a tamponade agent in the treatment of complicated retinal detachments. However, its benefits must be weighed against the risk of the complications associated with its use—cataract, band keratopathy, secondary glaucoma and potentially reduced visual acuity.1,2,3,4,5 The toxic effects of silicone oil are thought to be due to the migration and subsequent sequestration of silicone oil within ocular tissues as shown by several histopathological and clinical case series.6,7,8,9,10,11,12,13,14 Silicone oil has been observed in varied intraocular structures from the cornea to the retina, and also with progression through the optic nerve to the optic chiasm and brain.6,8,12 The presence of an associated inflammatory response in ocular tissue has also been documented, characteristically showing macrophages and giant cells laden with lipid vacuoles.13,14,15,16,17,18 In eyes that have undergone enucleation for advanced pathology, a degree of inflammation can be expected and it can be difficult to distinguish the inflammation due to the underlying pathology from that attributable to silicone oil. The nature and distribution of the inflammatory response associated specifically with silicone oil has yet to be characterised.14
This study was undertaken to analyse the distribution of silicone oil and its associated immunopathology in silicone‐oil‐filled globes, and to compare it with that seen in enucleated globes from patients with no history of silicone oil exposure. In addition, transmission electron microscopy was carried out on four of the silicone‐oil‐filled globes and on three of the control globes to determine the nature of silicone oil migration in the trabecular meshwork of the drainage angle.
Methods
This study was granted ethics approval by the Moorfields local research ethics committee (CHAD 1009).
Nine silicone‐oil‐filled eyes and seven control eyes enucleated after retinal reattachment surgery were analysed. Wherever possible, clinical case details were obtained from the referring hospital.
Processing/staining
Formaldehyde‐fixed specimens were embedded into paraffin wax using xylene as the ante‐medium. Tissue sections were stained by the haematoxylin and eosin sequence to assess general morphology. The immunohistochemical distribution of CD45RO (UCHL1) and CD45 (leucocyte common antigen) for T lymphocytes, CD20 (L26) for B lymphocytes, Mac 387 and CD68 (PGM1) for macrophages, Cam 5.2 for retinal pigment epithelium cells and glial fibrillary acidic protein (GFAP) for glial tissue was studied using a conventional alkaline phosphatase avidin‐biotin complex method. The antigens were visualised as the red final reaction product of Vector red (Vector Laboratories, Peterborough, UK). Appropriate negative (using non‐immune serum from the same species as the primary antibody and at the same protein concentration) and positive (using tissues known to express the antigen) controls were analysed. Table 1 outlines the primary antibodies used and the antigen retrieval information.
Table 1 Antigens studied.
| Antigen | Target | Antibody source | Antigen retrieval* | Antibody dilution |
|---|---|---|---|---|
| CD45RO | T lymphocytes | Dako | Heat mediated | 1:800 |
| CD45 | T lymphocytes | Dako | Heat mediated | 1:800 |
| CD20cy | B lymphocytes | Dako | Heat mediated | 1:600 |
| Mac 387 | Macrophages | Dako | Trypsin | 1:100 |
| CD68 | Macrophages | Dako | Trypsin | 1:100 |
| Cam 5.2 | RPE cells | BD Biosciences | Trypsin | 1:50 |
| GFAP | Glial tissue | Dako | Trypsin | 1:2000 |
GPAF, glial fibrillary acidic protein; RPE, retinal pigment epithelium.
Dako, Ely, UK; BD Biosciences, California, USA.
*Tissue sections were treated either by heat mediation, performed in an 800‐W microwave oven by heating 50 g/l urea in 50 mM TRIS‐HCl buffer, pH 9.5, for 10 min, followed by cooling for a further 25 min; or by trypsination, performed in a 37°C incubator by exposure to 1 g/l trypsin in 100 mM TRIS‐HCl buffer, pH 7.8, for 15 min.
A semiquantitative analysis of the cellular response and degree of silicone infiltration in intraocular tissues was undertaken. Grade + was used to indicate the presence of silicone oil or the presence of inflammatory cells. If silicone oil was observed in ocular tissues in >5 high‐powered fields, grade ++ was recorded. The diameter of one high‐powered field was 0.5 mm. Similarly, the presence of macrophages in >5 high‐powered fields was denoted as ++.
Transmission electron microscopy
Specimens originally prepared for routine wax embedding were dewaxed overnight in xylene, and then rehydrated to 10‐min rinses in 3×100% and 1×90%, 70% and 50% ethanol. After 2×10‐min changes of distilled water, blocks were fixed for 2 h in 1% osmium tetroxide, dehydrated to absolute alcohol by reversing the above process, passed through 2×20‐min changes of epoxy propane and left overnight in a 1:1 mixture of epoxy propane:araldite resin. After 12 h, the specimens were placed in 100% resin for 6 h with rotation, and then embedded and cured overnight in an oven at 60°C.
Semi‐thin (1 μm) sections for light microscopy and ultra‐thin (70 nm) sections for transmission electron microscopy were cut using a Leica Ultracut S microtome fitted with a diamond knife. Semi‐thin sections were stained with alcoholic toluidine blue. Ultra‐thin sections were contrasted by sequential staining with saturated uranyl acetate in 50% ethanol followed by lead citrate, and viewed and photographed in a JEOL 1010 transmission electron microscope (JEOL Ltd, Tokyo, Japan) operating at 80 kV.
Results
Silicone oil globes
Clinical details
The silicone‐oil‐filled group consisted of six males and three females. The average age at the time of enucleation was 40.4 years (range 11–86 years). The cause of initial presentation was rhegmatogenous retinal detachment (n = 3), penetrating eye injury (n = 4) and blunt trauma (n = 2). Silicone oil was present in all nine eyes at the time of enucleation. It was possible to document the duration of silicone oil tamponade in six of the nine cases: the average duration was 5 years (range 7 months–14 years).
Globes
Macroscopically, seven of the nine patients were aphakic at the time of enucleation and two patients were pseudophakic. A total retinal detachment was observed in two globes and a funnel retinal detachment in another two. In addition, a suprachoroidal haemorrhage was also noted in one globe.
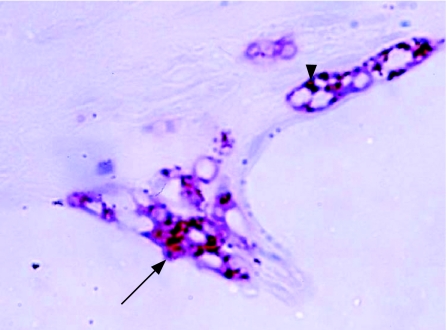
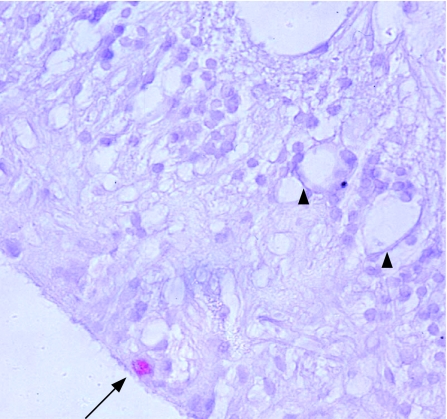
In the silicone‐oil‐filled globes, macrophage distribution was closely related to the distribution of silicone oil (table 2). The number of macrophages per high‐powered field generally reflected the degree of silicone oil distribution in the tissue. When this was not the case, there was a history of secondary pathology—for example, in one eye, an increased macrophage response in the anterior chamber was secondary to a perforated corneal ulcer and associated hypopyon. Similarly, one patient (number 4) had a membrane occluding the anterior chamber drainage angle with an associated macrophage and T lymphocyte infiltrate. Silicone oil was present in epiretinal membranes on the retinal surface in all nine globes, and in seven this was associated with a macrophage response. Giant cells were observed in epiretinal membranes in three globes. In four eyes silicone oil was found in the retina, with focal areas of retinal infiltration by macrophages occurring at retinectomy sites (either in the anterior frill of residual retina or at the posterior margin of the retinectomy), in grossly disorganised retina, or associated with the presence of subretinal silicone oil (fig 1). Silicone oil sequestered in the drainage angle, iris, ciliary body, retina and in epiretinal membrane was notable by the presence of small eccentric pigment granules in and adjacent to microglobules of oil. In lipid‐laden macrophages, these pigment granules were also observed in and surrounding the phagocytosed silicone microglobule (fig 1). These pigment granules were not observed in silicone oil found in the optic nerve (fig 2).
Table 2 Distribution of silicone oil in intraocular tissues of the silicone oil globes.
| Patient | ERM | Retina | Optic nerve | Ciliary body | Iris | Angle/TM | Cornea | Conjunctiva | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oil | M | Oil | M | Oil | M | Oil | M | Oil | M | Oil | M | Oil | M | Oil | M | |
| 1 | ++ | ++ | + | – | – | – | ++ | ++ | + | ++ | ++ | ++ | – | – | + | + |
| 2 | ++ | ++ | + | + | NA | NA | + | + | + | + | – | – | – | – | NA | NA |
| 3 | ++ | – | – | + | – | – | + | ++ | – | + | – | – | – | – | – | – |
| 4 | ++ | ++ | – | – | – | – | – | – | – | – | – | ++ | – | – | + | + |
| 5 | ++ | ++ | + | + | – | – | – | + | – | – | – | – | – | – | – | – |
| 6 | + | – | – | – | – | – | – | – | + | + | + | + | – | – | – | – |
| 7 | ++ | ++ | – | – | – | – | – | – | – | + | – | – | – | – | – | – |
| 8 | ++ | ++ | + | + | – | – | + | + | + | + | + | + | + | – | + | + |
| 9 | + | + | – | – | ++ | + | – | – | – | – | – | – | – | – | – | – |
ERM, epiretinal membrane; M, macrophage distribution; TM, trabecular meshwork; NA = specimen did not contain this structure; +, presence of silicone oil or inflammatory cells; ++, silicone oil was observed in ocular tissues in >5 high‐powered fields; –, absent.
Figure 1 Immunochemistry of the anterior edge of a retinectomy site in an enucleated globe after treatment with silicone oil. Intraretinal macrophages (CD68 antibody (red), haematoxylin counterstain) containing phagocytosed silicone oil (arrow) and eccentric pigment granules (arrowhead) are shown. Original magnification ×400.
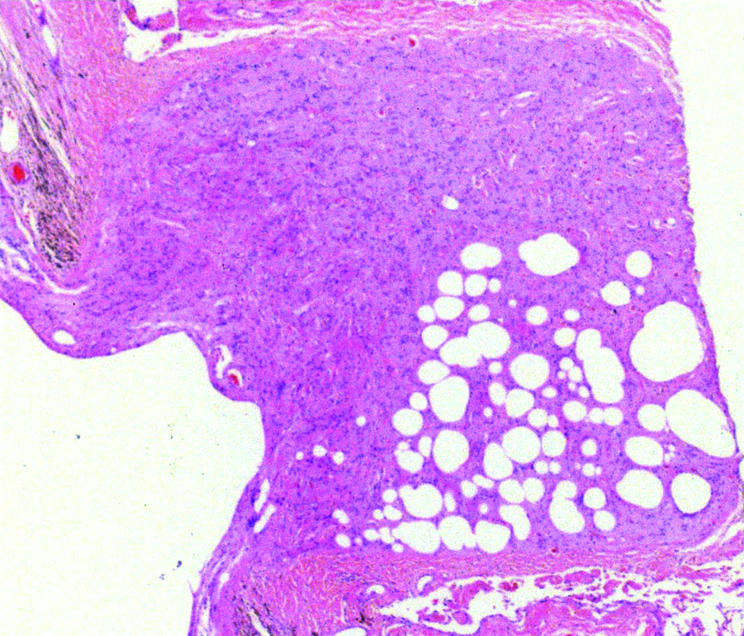
Figure 2 Light photomicrograph (haematoxylin and eosin) showing the presence of silicone oil in the cupped optic nerve head. Original magnification ×100.
Silicone oil, in association with macrophages, was present in the optic nerve in one patient who had a history of prolonged raised intraocular pressure due to secondary glaucoma 3 years after his initial surgery (fig 2). In this eye, silicone oil was observed in an epiretinal membrane but was not found elsewhere in the globe.
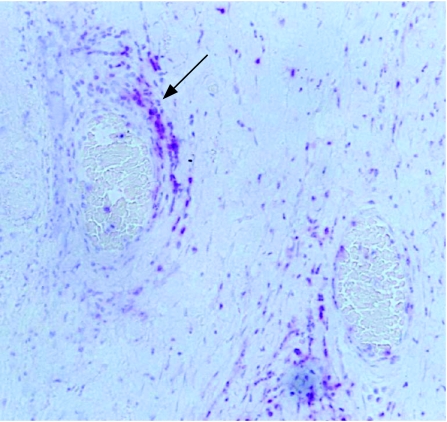
T lymphocytes were seen in five of the nine specimens. When present, T lymphocytes were found in cyclitic membranes (n = 1), proliferative vitreoretinopathy epiretinal membranes (n = 1), in membranes occluding the anterior chamber drainage angle (n = 2) and perivascularly in areas of inflammation (n = 4; fig 3). Perivascular populations of T lymphocytes were also found in the choroid and sclera (n = 4). T lymphocytes were not distributed in association with silicone oil. B lymphocytes were found in three of the nine specimens. When present, B lymphocyte populations were found together with T lymphocytes.
Figure 3 Immunohistochemistry of a control globe showing perivascular T lymphocytes (arrow: CD45 antibody (red), haematoxylin counterstain). Original magnification ×200.
The distribution of retinal GFAP was increased in all nine globes, being observed in all retinal layers. This did not differ from the control globes, where GFAP distribution was also increased in all seven eyes.
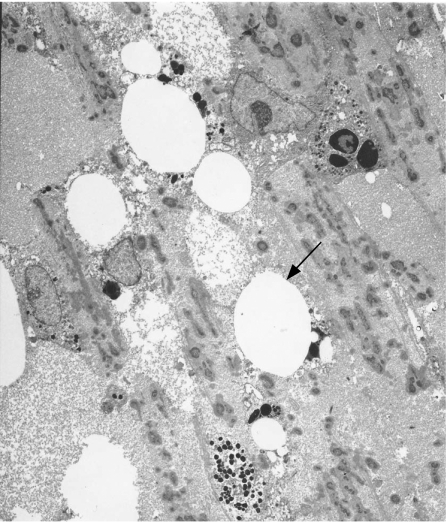
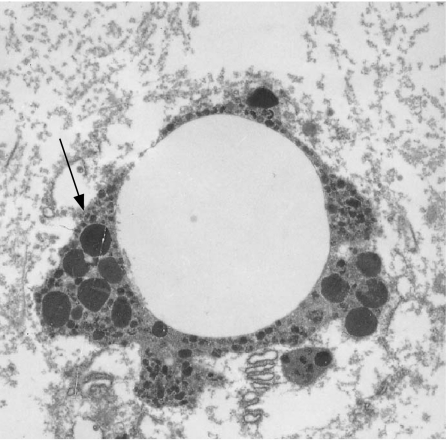
Transmission electron microscopy of the globes showed silicone oil in the trabecular meshwork in three of the four patients analysed (fig 4). Silicone oil was seen as discrete microglobules with small eccentric pigment granules (fig 5) on light microscopy.
Figure 4 Transmission electron micrograph showing the presence of silicone oil in the trabecular meshwork of the anterior chamber in a globe treated with silicone oil (arrow). Original magnification ×2000.
Figure 5 Transmission electron micrograph of a microglobule of silicone oil in the trabecular meshwork with eccentric pigment granules (arrow). Magnification ×5000.
Control globes
Clinical details
In the control group, five cases were male and two cases female. The average age at the time of enucleation was 45.7 years (range 23–71 years). The presenting pathologies were rhegmatogenous retinal detachment (n = 2), penetrating eye injury (n = 4) and blunt trauma (n = 1).
Globes
Macroscopically, one patient was aphakic and six patients were phakic. A total retinal detachment was noted in three globes and a funnel retinal detachment in one. A staphyloma and encirclement band was also observed in one globe.
In the control population, T lymphocytes were present in five of the seven globes. As in the case of the globes containing silicone oil, T lymphocytes were found in epiretinal proliferative vitreoretinopathy membranes (n = 2), cyclitic membranes (n = 2) and perivascularly (n = 3). B lymphocytes were found in association with T lymphocytes in two eyes. Macrophage infiltration was much less marked than that seen in the silicone‐oil‐filled eyes, and their distribution mirrored that of T lymphocytes. A scanty distribution of macrophages was also seen in areas of chronically detached retina and subretinal fluid (fig 6). In this group, optically empty spaces similar to those found in the silicone‐oil‐filled group were noted (fig 6) distributed in the retinal architecture and choroid. These spaces were not found in any other ocular structure, and could be distinguished from those found in the silicone‐oil‐filled globes by the absence of localised inflammatory cells and pigment granules.
Figure 6 Immunochemistry showing optically empty spaces (arrow heads) seen in the retina of a control globe. A single macrophage (CD68 antibody (red), haematoxylin counterstain) is also shown (arrow). Original magnification ×200.
Staining for GFAP was observed throughout the retinal layers in all seven control globes. Comparison of the silicone oil globes and the controls showed no differences in the distribution of GFAP.
Discussion
This study shows widespread infiltration of ocular tissue by silicone oil, in agreement with the findings of several previous reports.5,6,7,8,9,10,11,12,13,14 In addition, by immunohistochemical analysis we have shown that the intraocular inflammatory response associated with sequestered silicone oil differs from that seen in eyes with similar pathologies but without silicone oil. Examination of nine globes exposed to silicone oil found that the oil was closely associated with localised inflammation, suggesting that the inflammatory response was at least partly attributable to the presence of silicone oil rather than coexisting ocular pathology. Further evidence of this is the absence of an intense localised macrophage response in control eyes. Macrophages often contained phagocytosed silicone oil and seemed to remain viable despite large volumes of silicone oil in the cell, a finding that has been noted in other studies.17,19
Inflammatory responses mediated by T and B lymphocytes were not found in regions of silicone sequestration unless associated with other pathology—for example, cyclitic membranes, which suggests that these cells do not have a major role in the response to the presence of silicone oil. This was also supported by similar findings in the control globes where T and B lymphocyte infiltration was observed at sites of intraocular inflammation (epiretinal and cyclitic membranes and perivascularly) and in the subretinal fluid of chronic detachments.
Our study also supports the findings of two previous studies which observed that tissue infiltrated with silicone oil is usually confined to the retinal surface.20,21 In the silicone‐oil‐filled globes, intraretinal silicone oil was observed only when the retinal architecture was considerably disorganised—for example, at retinectomy sites or in areas where subretinal oil was noted. A similar finding was reported by Kirchhof et al22 in a case series of eight eyes in which intraretinal silicone oil was absent unless accompanied by subretinal oil. In addition, it has been hypothesised that the integrity of the outer limiting membrane may impede further silicone oil migration.23
Silicone oil was observed in the optic nerve of one globe in a patient with a history of raised intraocular pressure after oil injection. In a previously reported series of 74 globes, silicone oil was present in the optic nerve in 14 cases (24%), and in 10 of the 14 eyes with silicone oil in the optic nerve, there was a history of raised intraocular pressure after silicone oil injection,6 a finding that has also been documented in other case reports.9,24 Migration of silicone oil into the optic nerve may be responsible for cases of unexplained visual loss before and after silicone oil removal, which have recently been reported.25,26
Our study also showed ultrastructural evidence of silicone in the trabecular meshwork in four patients, and immunohistochemically we found a macrophage response associated with oil in the meshwork. A previous study failed to show evidence of silicone oil or macrophages in the drainage angle in two patients with secondary glaucoma attributed to silicone oil.27 The differences between these studies and the drainage angle findings may be related to sampling artefacts depending on which part of the angle is studied. Overall, our results suggest trabecular infiltration by silicone oil and an associated trabeculitis. These changes are likely to persist even after oil removal, and may well account for the chronically raised intraocular pressure seen in eyes that have previously had intraocular silicone oil.
It should be noted, however, that the globes studied have in general advanced intraocular pathology (as is the case in previous similar series), and the infiltration of silicone oil we have found may not reflect its distribution in eyes with less advanced pathology and shorter durations of silicone oil exposure. We have noted in a series of retinectomy specimens (where pathological changes are less advanced) that silicone infiltration into the retina was uncommon and generally confined to regions of disorganised and gliotic retina (data submitted). Nevertheless, the widespread distribution of silicone oil suggests its potential (given appropriate intraocular conditions such as retinal scarring) to cause ongoing pathology.
The difficulty of having complete certainty in detecting silicone oil in intraocular tissue deserves emphasis. Silicone oil is removed during the routine processing of tissue for histological analysis, and therefore its presence appears as optically empty spaces in tissue sections. Similar findings are also found in tissues that have not been exposed to silicone oil,21,22,23 and may be due to tissue oedema, gliosis or processing artefacts. In long‐standing retinal detachment or other advanced retinal pathology, areas of retinal oedema may be seen as rounded, optically empty spaces, and can be very difficult to distinguish from silicone oil. In the current study, the presence of silicone oil was closely related to an inflammatory response that can suggest the presence of foreign material. We observed that optically empty spaces due to silicone oil were also associated with small eccentric granules of pigment both within and surrounding the oil globule, apparently related to the macrophage‐mediated inflammatory response as shown in fig 1. This finding was not seen in optically empty spaces in the control specimens (fig 6).
In conclusion, this study has shown that silicone oil may be sequestered in varied ocular tissues—notably iris, ciliary body, trabecular meshwork, retina and epiretinal membranes. In globes with advanced pathology, silicone oil seems to initiate a localised inflammatory response mediated by macrophages, whereas other associated ocular pathology is characterised by a marked T lymphocyte infiltrate, with some involvement of B lymphocytes and macrophages. Our findings suggest that intraretinal oil usually occurs as a result of persistent retinal detachment or retinal injury; however, sequestration of silicone oil in other tissues, notably the drainage angle, ciliary body and optic nerve, may occur despite retinal reattachment surgery. Removal of silicone oil may not reverse the inflammatory cycle initiated by its injection, and explains the ability of silicone oil to cause multiple long‐term sequelae.
Abbreviations
GFAP - glial fibrillary acidic protein
Footnotes
Competing interests: None declared.
References
- 1.Leaver P. Complications of intraocular silicone oil. Retina. 2nd edn. Ryan SJ, Glaser BM, eds. St Louis, MO: Mosby, 1994
- 2.Watzke R C. Silicone retinopoesis for retinal detachment: a long‐term clinical evaluation. Arch Ophthalmol 196777185–196. [DOI] [PubMed] [Google Scholar]
- 3.Abrams G W, Azen S, McCuen B.et al Vitrectomy with silicone oil or long‐acting gas in eyes with severe proliferative vitreoretinopathy: results of additional long‐term follow up. Silicone Study Report 11. Arch Ophthalmol 1997115335–344. [DOI] [PubMed] [Google Scholar]
- 4.Foulks G, Hatchell D, Proia A.et al Histopathology of silicone oil keratopathy in humans. Cornea 19911029–37. [PubMed] [Google Scholar]
- 5.Honavar S, Goyal M, Majji A.et al Glaucoma after pars plana vitrectomy and silicone oil injection for complicated retinal detachments. Ophthalmology 1999106169–177. [DOI] [PubMed] [Google Scholar]
- 6.Budde M, Cursiefen C, Holbach L M.et al Silicone oil‐associated optic nerve degeneration. Am J Ophthalmol 2001131392–394. [DOI] [PubMed] [Google Scholar]
- 7.Srinivasan A, Singh A K, Desai S P.et al Foreign body episcleral granulomas complicating intravitreal silicone oil tamponade – a clinicopathological study. Ophthalmology 20031101837–1840. [DOI] [PubMed] [Google Scholar]
- 8.Eckle D, Kampik A, Hintschich C.et al Visual field defect in association with chiasmal migration of intraocular silicone oil. Br J Ophthalmol 200589918–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ni C, Wang W, Albert D.et al Intravitreous silicone injection – histopathological findings in a human eye after 12 years. Arch Ophthalmol 19831011399–1401. [DOI] [PubMed] [Google Scholar]
- 10.Chung J, Spaide R. Intraretinal silicone oil vacuoles after macular hole surgery with internal limiting membrane peeling. Am J Ophthalmol 2003136766–767. [DOI] [PubMed] [Google Scholar]
- 11.Donahue S P, Friberg T R, Johnson B L. Intraconjunctival cavitary inclusions of silicone oil complicating retinal detachment repair. Am J Ophthalmol 1992114639–640. [DOI] [PubMed] [Google Scholar]
- 12.Eller A, Friberg T, Mah F. Migration of silicone oil into the brain:a complication of intraocular silicone oil for retinal tamponade. Am J Ophthalmol 2000129685–688. [DOI] [PubMed] [Google Scholar]
- 13.Shaikh S, Egbert, Goldblumet al Granulomatous local cell reaction to intravitreal silicone. Arch Ophthalmol 20001181133–1134. [PubMed] [Google Scholar]
- 14.Knorr H L J, Seltsam A, Holbach L M.et al Intraocular silicone oil: a clinicopathological study of 36 enucleated eyes. Ophthalmologe 199693130–138. [PubMed] [Google Scholar]
- 15.Parmley V C, Barishak Y R, Howes E L.et al Foreign‐body giant cell reaction to liquid silicone. Am J Ophthalmol 1986101680–683. [DOI] [PubMed] [Google Scholar]
- 16.Heidenkummer H P, Messmer E M, Kampik A. Recurrent vitreoretinal membranes during intravitreal silicone oil tamponade. Morphological and immunohistochemical investigations. Ophthalmologe 199693121–125. [PubMed] [Google Scholar]
- 17.Betis F, Leguay J M, Hofman P. Multinucleated giant cells in periretinal silicone granulomas are associated with progressive proliferative vitreoretinopathy. Eur J Ophthalmol 200313634–641. [DOI] [PubMed] [Google Scholar]
- 18.Jerden J, Pepose J, Michels R.et al Proliferative vitreoretinopathy membranes – an immunohistochemical study. Ophthalmology 198996801–810. [DOI] [PubMed] [Google Scholar]
- 19.Bornfeld N, El‐Hifnawi E, Laqua H. Ultrastructural characteristics of preretinal membranes from human eyes filled with silicone oil. Am J Ophthalmol 1987103770–775. [DOI] [PubMed] [Google Scholar]
- 20.Lewis H, Burke J M, Abrams G W.et al Perisilicone proliferation after vitrectomy for proliferative vitreoretinopathy. Ophthalmology 198895583–591. [DOI] [PubMed] [Google Scholar]
- 21.Eckardt C, Nicolai U, Czank M.et al Identification of silicone oil in the retina after intravitreal injection. Retina 199212S17–S22. [DOI] [PubMed] [Google Scholar]
- 22.Kirchhof B, Tavakolian H, Heimann K. Histopathological findings in eyes after silicone oil injection. Graefe's Arch Clin Exp Ophthalmol 198622434–37. [DOI] [PubMed] [Google Scholar]
- 23.Shikishima K, Ohki K, Machi N.et al Effects and distribution of intravitreally or subretinally injected silicone oil identified in rabbit retina using osmium tetroxide method. Jpn J Ophthalmol 199236469–478. [PubMed] [Google Scholar]
- 24.Shields C, Eagle R. Pseudo‐Schnabel's cavernous degeneration of the optic nerve secondary to intraocular oil. Arch Ophthalmol 1989107714–717. [DOI] [PubMed] [Google Scholar]
- 25.Newsom R S, Johnston R, Sullivan P M.et al Sudden visual loss after removal of silicone oil. Retina 200424871–877. [DOI] [PubMed] [Google Scholar]
- 26.Herbert E N, Habib M, Steel D.et al Central scotoma associated with intraocular silicone oil tamponade develops before oil removal. Graefe's Arch Clin Exp Ophthalmol 2006244(2)248–252. [DOI] [PubMed] [Google Scholar]
- 27.Cvenkel B, Zupan M, Hvala A. Transmission electron microscopic analysis of trabecular meshwork in secondary glaucoma after intravitreal silicone oil injection. Int Ophthalmol 19972043–47. [DOI] [PubMed] [Google Scholar]