Abstract
Aim
To evaluate the effect of verteporfin photodynamic therapy (PDT) on endostatin with regard to expression of vascular endothelial growth factor (VEGF) in human choroidal neovascular membranes (CNVs) secondary to age‐related macular degeneration.
Methods
A retrospective review of an interventional case series of 68 patients who underwent removal of CNV. 29 patients were treated with PDT 3–655 days before surgery. 39 CNVs without previous treatment were used as controls. CNVs were stained for CD34, CD105, Ki‐67, cytokeratin 18, endostatin, E‐selectin and VEGF. “Predominance score of VEGF over endostatin” (mean) was defined as the difference between VEGF and endostatin staining scores.
Results
In four CNVs treated by PDT 3 days previously, PS was significantly higher in the retinal pigment epithelium (mean = 2.5, p = 0.006) and stroma (mean = 2, p = 0.015) than in the control group (mean = 0). At longer post‐PDT intervals, PS was significantly decreased in the retinal pigment epithelium (mean = 0, p = 0.019) and stroma (mean = 0, p = 0.015). Proliferative activity was high (p = 0.023), but mostly related to inflammatory cells. PDT did not influence E‐selectin expression significantly.
Conclusions
VEGF predominance over endostatin early after PDT might contribute to enhanced angiogenic activity associated with recurrences. Strategies upregulating or replacing endostatin early after PDT might increase the effectiveness of PDT.
Neovascular age‐related macular degeneration (AMD) is the leading cause of visual loss among elderly in the western world.1 Photodynamic therapy (PDT) with verteporfin (Visudyne, Ciba Vision Corporation, Duluth, Georgia, USA) is proved to be beneficial in clinical trials.2,3,4,5,6 This benefit, however, is limited by high recurrence and high retreatment rate.2,3,4,5,6 Choroidal neovascular membrane (CNV) extraction alone is not favoured by submacular surgery trials,7 but macular translocation was suggested as an option for patients who did not profit from prior PDT.8,9,10,11 Nevertheless, antiangiogenic treatments12,13,14 are now changing the philosophy to a modulative rather than ablative treatment. As adjuvant to PDT, they may increase its efficiency. To establish a beneficial combined treatment, it is essential to understand the influence of PDT on the targeted tissue.
Vascular endothelial growth factor (VEGF), a promoter of CNV development,15,16 is upregulated after verteporfin PDT.17 Neovascularisation, as in CNV, occurs due to an impaired local balance between angiogenesis promoters and inhibitors.18 VEGF predominates over pigment‐endothelium derived factor, which is an endogeneous angiogenesis inhibitor.19 Endostatin, a C‐terminal fragment of collagen XVIII, is another potent endogenous angiogenesis inhibitor in CNV.20,21
In retinal pigment epithelium (RPE) and choriocapillaris of human eyes with AMD, decreased expression of endostatin was suggested to predispose to CNV formation.22 Laser‐induced CNV lesions were significantly bigger in mutant mice lacking collagen XVIII/endostatin than in the control animals.23 Endostatin downregulates many angiogenic genes including those of VEGF, and upregulates several antiangiogenic genes.24 Intraocular expression of endostatin reduces VEGF‐induced retinal permeability and neovascularisation.25
In order to understand potential interactions, we evaluated the expression and chronological sequence of VEGF, endostatin and E‐selectin, as well as vascularisation and proliferative activity in CNV excised after different time intervals after PDT.
Methods
Subjects and treatments
We retrospectively reviewed 68 eyes of 68 consecutive patients with AMD who were treated with macular translocation at five distinct surgical sites between 1997 and 2005. In all, 29 patients received surgery after verteporfin PDT. Table 1 summarises the clinical characteristics of the patients treated with PDT before macular translocation.
Table 1 Clinical characteristics of patients treated with PDT before surgical removal of the CNV.
| Case | Eye | Age/sex | CNV type | Number of PDT | Time to surgery from the initial PDT to final PDT |
|---|---|---|---|---|---|
| 1 | L | 76/M | Classic | 1 | 3 days |
| 2 | R | 78/F | Classic | 1 | 3 days |
| 3 | L | 54/M | Predominantly classic | 2 | 113/3 days |
| 4 | L | 84/M | Classic | 1 | 3 days |
| 5 | R | 74/F | Occult | 1 | 21 days |
| 6 | L | 83/M | Classic | 1 | 34 days |
| 7 | L | 85/F | Classic | 1 | 37 days |
| 8 | R | 73/F | Occult | 3 | 208/138/40 days |
| 9 | L | 78/F | Predominantly classic | 2 | 3 months/54 days |
| 10 | L | 79/M | Classic | 1 | 55 days |
| 11 | R | 80/F | Classic | 2 | 172/69 days |
| 12 | L | 66/F | Occult | 1 | 83 days |
| 13 | L | 77/M | Minimally classic | 1 | 84 days |
| 14 | R | 79/F | Predominantly classic | 1 | 88 days |
| 15 | L | 70/M | Predominantlyclassic | 1 | 92 days |
| 16 | R | 93/M | Classic | 2 | 95 days |
| 17 | L | 76/F | Occult | 1 | 105 days |
| 18 | L | 87/M | Predominantly classic | 1 | 108 days |
| 19 | R | 71/M | Classic | 1 | 112 days |
| 20 | L | 81/M | Classic | 2 | 213/131 days |
| 21 | R | 70/F | Classic | 2 | 151/132 days |
| 22 | L | 78/F | Classic | 3 | 344/222/146 days |
| 23 | L | 77/M | Classic | 3 | 329/245/147 days |
| 24 | L | 72/M | Predominantly classic | 2 | 232/156 days |
| 25 | R | 79/F | Predominantly classic | 1 | 171 days |
| 26 | R | 74/F | Haemorrhagic | 1 | 246 days |
| 27 | L | 81/F | Classic | 6 | 824/300 days |
| 28 | L | 73/F | Classic | 4 | 677/558/467/383 days |
| 29 | L | 77/F | Predominantly classic | 7 | Unknown*/772/655 days |
CNV, choroidal neovascularisation membrane; F, female; L, left; M, male; PDT, photodynamic therapy; R, right.
*Time of 1st–5th PDT session unknown.
Treatment options, including observation, conventional thermal laser photocoagulation, PDT retreatment, intravitreal triamcinolone injection and macular translocation with 360° retinotomy, were discussed with the patients. Surgical intervention was offered (a) when visual acuity was <20/200, which is the minimum visual acuity to recommend initial PDT according to the Treatment of Age‐Related Macular Degeneration with Photodynamic Therapy investigation2,3; (b) when visual deterioration progressed after initial PDT; (c) when the patient refused PDT or re‐treatment with PDT due to continuous visual deterioration in the fellow eye despite PDT; and (d) when PDT was impossible due to recurrent or massive submacular haemorrhage. In four cases, verteporfin‐PDT was performed 3 days before surgery with the intention to reduce bleeding from the lesion site at the time of surgical extraction. Three of these cases were not eligible for the initial PDT according to Treatment of Age‐Related Macular Degeneration with Photodynamic Therapy criteria, as their visual acuity varied between 4/200 and 5/200.2,3,4,5,6 The fourth patient opted to proceed with surgery rather than PDT retreatment as he experienced a decrease in visual acuity 3 months after the initial PDT.
Written informed consent was obtained from each patient after the experimental nature of the procedure and risks and benefits of all therapeutical options had been fully explained. The study followed the guidelines of the declaration of Helsinki as revised in Tokyo and Venice. The study and the histological analysis of the specimens were approved by the local Institutional Review Board.
Tissue preparation
Within minutes after surgery, excised CNV were fixed in 3.7% formalin and subsequently embedded in paraffin. Each specimen was serially mounted on poly‐L‐lysine‐coated glass slides (Dako, Glostrup, Denmark).
Immunohistology
Serial sections were deparaffinised and rehydrated with a graded series of alcohol. For cytokeratin 18 and endostatin, antigen retrieval was accomplished by proteolytic digestion with 0.5% protease XXIV (Sigma, St Louis, Missouri, USA), whereas proteinase K (Dako) was used for VEGF. For Ki‐67, CD34, CD105 and E‐selectin, antigen retrieval was with heat treatment in citrate buffer (0.01 M, pH 6.0).
Immunohistochemical analysis with primary antibodies for human CD105 (mouse, monoclonal antibody (Mab), Clone SN6h, Dako), CD34 (mouse, Mab, Immunotech, Hamburg, Germany), Ki‐67 (mouse, Mab, Clone Ki‐S5, Dako), cytokeratin 18 (mouse, Mab, Progen, Heidelberg, Germany) and E‐selectin (mouse, Mab, Novocastra, UK) was performed using horseradish peroxidase as described previously.17 CD34, CD105, cytokeratin 18 and Ki‐67 were used to label endothelial cells, activated endothelial cells, RPE and proliferating cells, respectively.26,27,28,29
Immunohistochemical analysis for VEGF and endostatin was performed by the alkaline‐phosphatase method as described previously,17 using an anti‐human VEGF‐A antibody (mouse, Mab, clone C‐1; Santa Cruz Biotechnology, Santa Cruz, California) and an anti‐human endostatin antibody (rabbit, polyclonal, Dianova GmbH, Hamburg). Haematoxylin (Chemmate, Code S2020, Dako) was used for counterstaining.
For negative controls, primary antibodies were either substituted by appropriate normal sera or omitted.
Analysis
Serial sections from a specimen were analysed independently by two blinded observers (OT, SG) by light microscopy.
Each specimen was documented with a digital microscope (Axioskop, Zeiss, Oberkochen, FRG) connected to a digital camera (HC‐300Z, Fujix, Japan). Area of each specimen was measured using the appropriate hardware and software (AxioVision, V.3.1, Carl Zeiss, Göttingen, Germany).
All Ki‐67 positive nuclei all over the section were counted in each specimen. Proliferative activity in a specimen was determined quantitatively by the ratio of the total number of Ki‐67 positive nuclei in CNV to the total area of the membrane (mm2).
Immunoreactivity for VEGF, endostatin and E‐selectin were analysed separately in RPE–Bruch's membrane complex, vessels and stroma. A grading scheme indicating degree of staining was used: grades 3, 2, 1 and 0 were assigned to indicate intense (70–100% positive cells), moderate (40–69% positive cells), weak labelling (1–39% positive cells) and absence of any staining, respectively. Owing to the inadequate pretreatment stability of some sections, E‐selectin expression was evaluated in 22 CNVs treated without PDT and in 26 CNVs treated with PDT.
“Predominance score of VEGF over endostatin” (PS) was defined for RPE, vessels and stroma of each membrane separately, calculating the difference between VEGF and endostatin staining scores.
Intensity of VEGF, endostatin and E‐selectin immunostaining, PS and proliferative activity of the defined subgroups were comparatively analysed with Mann–Whitney U test, p⩽0.05 was considered significant.
Results
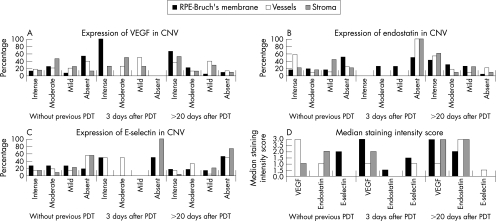
Figure 1 summarises the frequency of VEGF, endostatin and E‐selectin immunoreactivity intensity, and corresponding median staining intensity scores in untreated and PDT‐treated CNV.
Figure 1 Graphs showing vascular endothelial growth factor (VEGF) (A), endostatin (B) and E‐selectin (C) immunostaining intensity and median staining intensity scores (D) in choroidal neovascular membranes (CNVs) without photodynamic therapy (PDT) and CNV extracted at 3 days and >20 days after PDT. VEGF, endostatin and E‐selectin immunostaining in retinal pigment epithelium (RPE)‐Bruch's membrane, vessels and stromal cells were evaluated separately and semiquantitatively as intense (70–100% positive cells), moderate (40–69% positive cells), mild (1–39% positive cells) or absent. Staining scores of 3, 2, 1 and 0 were assigned to “intense”, “moderate”, “mild” and “absent” intensity of staining, respectively.
Characterisation of CNV without prior PDT
All but one membrane were vascularised as evidenced by CD34 and CD105 positive vessels (fig 2A,B). RPE cells were found in all specimens.
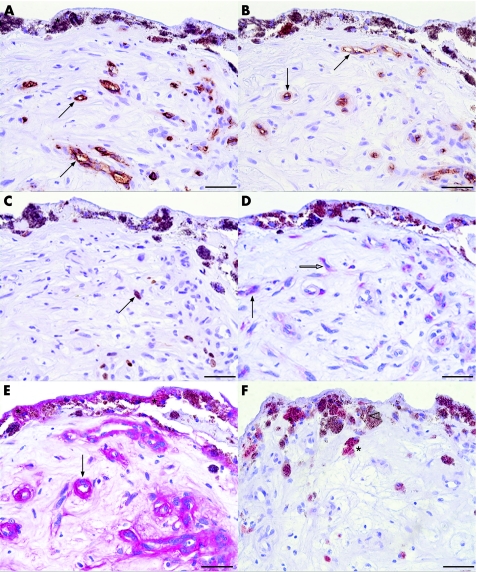
Figure 2 Photomicrographs of surgically excised choroidal neovascular membranes without prior photodynamic therapy. The specimens were probed with antibody against CD34 (A), CD105 (B) and Ki‐67 (C) stained with 3‐diaminobenzidine, resulting in a brown chromogen; vascular endothelial growth factor (VEGF) (D) and endostatin (E) stained with red chromogen; and E‐selectin (F) with 3‐amino‐9‐ethyl carbazole. Haematoxylin was used as a counterstain. CD34 (A) and activated endothelial cell marker CD105 (B) are selectively expressed in vascular structures (arrow). The brown chromogen can be distinguished from the melanin granula (asterisk) contained in pigmented cells. Several cell nuclei express the proliferation marker Ki‐67 (C, arrow). In the serial section of the same specimen (D), VEGF staining was detected within endothelial cells (arrow) and stromal cells (white arrow). In a serial section probed with endostatin, retinal pigment epithelium (RPE)‐Bruch's membrane (asterisks) and vessels (arrow) express endostatin (E). (F) Some RPE cells (asterisk) display E‐selectin immunoreactivity, whereas some RPE cells are not immunoreactive (white arrowhead). Scale bar: 50 μm.
Proliferative activity varied between 0 and 1959.27 Ki‐67 positive nuclei/mm2 (median: 53.698; fig 2C).
VEGF was absent in the RPE of 53.8% (21/39) of the specimens. In only 12.8% (5/39) of the CNV was VEGF strongly expressed in RPE. VEGF was detected in endothelial cells and stromal cells in 60.5% (23/38) and 95% (33/38) of the membranes, respectively (figs 1A,D and 2D).
Endostatin was found in RPE–Bruch's membrane complex and in vessels in 48.7% (19/39) and 76.3% (29/38) of the specimens, respectively. Within stroma, endostatin was present in fibroblast‐like and inflammatory cells in 79.5% (31/39) of the membranes (figs 1B,D and 2E).
Endothelial cells, RPE and stromal cells disclosed E‐selectin in different intensities in 45.5% (10/22), 81.8% (18/22) and 45.5% (10/22) of the membranes, respectively (figs 1C,D and 2F).
Characterisation of CNV treated by PDT
3 days after PDT
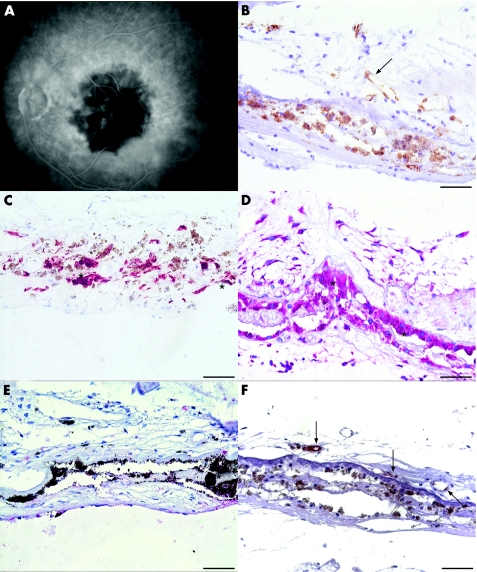
A hypofluorescence suggesting non‐perfusion of the irradiated area and CNV was seen in the early phases of angiography (fig 3A). Late phases of fluorescein angiography showed hyperfluorescence and leakage at fovea consistent with choroidal ischaemia (data not shown).
Figure 3 Photomicrographs of choroidal neovascular membranes (CNV) membrane (case 4, table 1) extracted 3 days after photodynamic therapy. Early phase of fluorescein angiography (A) on the day of surgery displays non‐perfusion of the CNV and laser spot area. The serial sections were probed with CD34 (B), cytokeratin 18 (C), vascular endothelial growth factor (VEGF) (D), endostatin (E) and E‐selectin (F). Some vessels depicted by the brown chromogen are patent, but are still lined with damaged endothelial cells (B, arrow). Retinal pigment epithelium (C, asterisks) are strong positive for VEGF (D, asterisks), but not immunoreactive for endostatin (E, asterisk). Endostatin immunoreactivity is absent in CNV (E). Endothelial cells express E‐selectin (F, arrows). Scale bar: 50 μm.
Immunohistology with CD34 and CD105 disclosed mostly occluded but several patent vessels lined with damaged endothelial cells (fig 3B).
In all membranes (n = 4), cytokeratin 18 positive RPE (fig 3C) displayed an intense staining for VEGF (figs 1A and 3D), which was significantly higher than that in the control group (p = 0.003; fig 1D). Endostatin was found in the RPE–Bruch's membrane complex of only two membranes (figs 1B and 3E). Consequently, PS in RPE–Bruch's membrane complex was significantly higher than that in the control group (PS = 2.5 and 0, respectively, p = 0.006).
VEGF expression in endothelial cells and stroma varied in intensity (fig 1A). However, none of the specimens displayed endostatin in vessels or stroma (figs 1B and 3E). Endostatin expression in vessels and stroma was significantly weaker than control CNV (fig 1D, p = 0.037 and 0.003, respectively). Consequently, PS in stroma was significantly higher than in the control group (PS = 2 and 0, respectively, p = 0.005).
E‐selectin was expressed either in RPE or in endothelial cells (figs 1C,D and 3F).
Ki‐67 was completely negative in two cases (median proliferative activity 4 85 505, range 0–78.758 nuclei/mm2).
Post‐PDT intervals longer than 3 days
Fluorescein angiography disclosed hyperfluorescent membranes with leakage in late phases (data not shown).
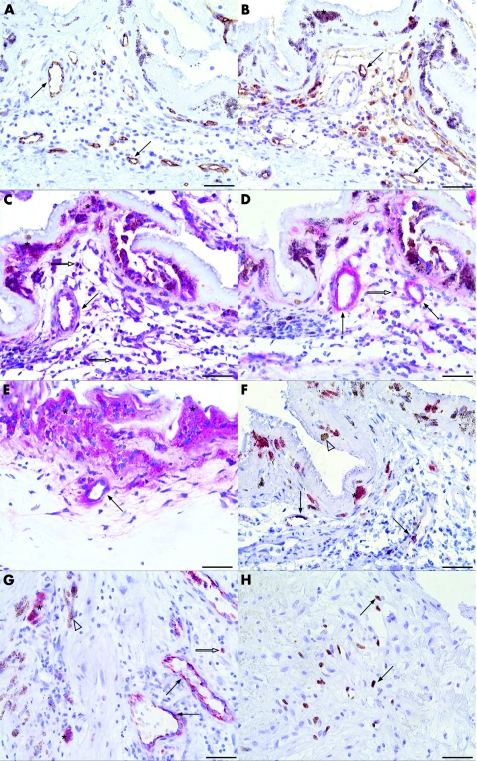
Patent vessels lined with CD34 positive endothelial cells with prominent nuclei were detected in all but one membrane (fig 4A). Strong CD105 immunoreactivity reflected very vital and active endothelial cells. Some CD105 negative endothelial cells were also detected (fig 4B). VEGF was detected in endothelial cells in 21 (87%) samples (fig 4C). Vessels displayed endostatin in 82.8% (19/24) of CNVs (40%) in stronger intensity than in CNVs extracted 3 days after PDT (p = 0.007; figs 1B,D and 4D,E). E‐selectin was seen in endothelial cells in 50% (11/22) of the specimens (figs 1C and 4F,G).
Figure 4 Photomicrographs of choroidal neovascular (CNV) membranes extracted 55 and 383 days after PDT from case 10 (A–D, F, H) and case 28 (E, G) in table 1. The sections were probed with CD34 (A), CD105 (B), vascular endothelial growth factor (VEGF) (C), endostatin (D, E), E‐selectin (F, G) and Ki‐67 (H). Most of the vessels depicted by the brown chromogen are patent and lined with healthy activated endothelial cells (A, B, arrows). The brown chromogen can be distinguished from the melanin granula (asterisk) contained in pigmented cells. (C) Retinal pigment epithelium (RPE; asterisk), endothelial cells (arrows) and stromal cells (white arrow) display VEGF (red chromogen) strongly. (D, E) Endostatin expression becomes stronger in RPE–Bruch's membrane complex (asterisk), vessels (arrows) and stromal cells (white arrow) as the time interval after photodynamic therapy (PDT) increases. (F, G) Endothelial cells (arrows), some stromal cells (white arrow) and some RPE cells (asterisk) express E‐selectin, whereas some RPE cells are E‐selectin negative (white arrowhead). (H) Many Ki‐67‐expressing proliferating cells were detected in the serial section of the CNV from case 10 (arrows, brown chromogen). Scale bar: 50 μm.
All but one membrane (case 17 in table 1) were extracted with RPE as evidenced by cytokeratin 18 staining. Strong VEGF staining in RPE in 66.7% (16/24; figs 1A and 4C) of the specimens persisted to be significantly higher than in the control group (fig 1D, p<0.001). Endostatin expression in RPE (in 23/24 specimens) was significantly higher (figs 1B and 4D,E, p = 0.026), and, consequently, PS in RPE was significantly lower than in CNV extracted 3 days after PDT (fig 1D, PS = 0 and 2.5, respectively, p = 0.002). RPE expressed E‐selectin in 47.8% (11/23) of the specimens (figs 1C and 4F,G).
Stromal cells displayed VEGF and endostatin in 92% (23/25) of the membranes (figs 1A,B and 4C–E). Endostatin in stroma was considerably stronger (fig 1D, p = 0.002) and PS in stroma (PS = 0) was significantly lower than in CNV treated by PDT 3 days preoperatively (PS = 2, p = 0.015). Stromal cells disclosed E‐selectin in 26.1% (6/23) of the specimens (figs 1C and 4F–G).
No noticeable change was found in pattern of E‐selectin expression in any component of the CNV subgroups examined.
Proliferative activity (median 114.125, range 0–955.235) was significantly higher than both CNVs extracted 3 days after PDT (p = 0.023) and the control group (p = 0.02; fig 4H).
Discussion
PDT is based on the formation of free radicals and reactive oxygen intermediates which damage endothelial cells and lead to a selective occlusion of the targeted vessels.30,31,32 In >90% of the cases, however, a recurrence is seen within 3 months.2,3,4,5,6 Enhanced VEGF expression17 and predominance over pigment epithelium‐derived factor19 might contribute to this rebound effect. Herein, we investigated the potential involvement of E‐selectin and endostatin in this process.
E‐selectin is a prerequisite for the antiangiogenic effect of endostatin.33 Similar to the previous study,34 we did not see a noticeable change in E‐selectin expression after PDT.
By contrast, endostatin was considerably weaker in intensity in vessels and stroma 3 days after PDT compared with the untreated specimens. As a consequence, VEGF predominates over endostatin in RPE–Bruch's membrane and in stroma early after PDT. A hypoperfusion‐related hypoxia and the release of ROI after PDT might have upregulated VEGF expression by RPE.35,36,37,38,39,40,41 To our knowledge, the effect of PDT and hypoxia on ocular endostatin expression is unknown. Reduction of endostatin, however, was suggested to have an active role in hypoxia‐driven angiogenesis elsewhere.42,43
Endostatin has important implications in inhibition of angiogenesis and ischaemia‐induced neovascularisation.44 In early phases of angiogenesis, it inhibits VEGF‐induced endothelial cell migration, stabilises newly formed endothelial tubes,45,46 downregulates VEGF expression and blocks the VEGF/Flk‐1 pathway.47,48 Decreased levels of endostatin with consequent VEGF predominance 3 days after PDT may, therefore, have a permissive role in the reactive angiogenic process.
In CNV extracted at longer time intervals after PDT, endostatin expression was markedly enhanced in RPE–Bruch's membrane complex, endothelial cells and stroma. Zatterstrom et al49 suggested that activated endothelial cells secrete proteolytic enzymes that release active endostatin from collagen XVIII in vascular basement membranes.50 This might explain why endostatin was diminished in the early post‐PDT period when endothelial cells were severely damaged,30,31,32 but enhanced thereafter when healthy endothelial cells were found. Increased infiltration with leucocytes and macrophages (unpublished data) producing proteolytic enzymes may also contribute to enhanced release of endostatin.51 As a consequence, VEGF predominance over endostatin is diminished or abolished at a certain phase of the revascularisation process after PDT. High proliferative activity, patent vessels with activated endothelial cells and persisting leakage in fluorescein angiography in these specimens seem paradoxical, but may be the result of the previous imbalance with VEGF predominance. It has been shown previously that even temporarily enhanced VEGF expression by RPE was sufficient for increased vascular leakage and development of CNV.52 Therefore, insufficient counterbalance of VEGF early after PDT seems to have restarted the angiogenic cascade. Inhibition of vessel growth by endostatin would have required an early and substantial presence of endostatin.53,54
Endostatin inhibits experimental CNV,55,56,57 and its expression can be upregulated by orally administered drugs.58 Up regulation or exogeneous delivery of endostatin early after PDT might therefore be a useful strategy to increase the efficacy of PDT. A concomitant anti‐VEGF treatment having a synergistic effect59,60 should be even more potent.
The sequence of histopathological changes in CNV after PDT probably reflects not the cause but the natural process of CNV formation. Enhanced endostatin expression in the late stages of angiogenesis possibly contributes to the involution process. The exact time of CNV onset is mostly unknown and the age of the membranes at the time of PDT or surgery cannot be assessed. PDT, however, gives an artificial but accurate “time zero” that enables a chronological analysis of the revascularisation process and the humoral and cellular mechanisms involved. An absolute quantification of the mRNA or protein expression by real‐time polymerase chain reaction or western blot in further studies will probably supply additional valuable information.
To our knowledge, this is the first report of clinicopathological correlation related to the expression of endostatin in human CNV treated by verteporfin PDT. The proper interpretation of this study is limited by a potential negative selection of PDT‐treated cases based on the retrospective and non‐randomised characteristics. Additionally, the maturity and angiogenic activity of the specimens probably vary within and between groups and cannot be unified precisely. Nevertheless, our findings show that angiogenic VEGF predominates over antiangiogenic endostatin early after PDT. An exogenous or endogenous increase of endostatin in this phase is a potential approach that merits investigation.
Abbreviations
AMD - age‐related macular degeneration
CNV - choroidal neovascular membrane
Mab - monoclonal antibody
PDT - photodynamic therapy
RPE - retinal pigment epithelium
VEGF - vascular endothelial growth factor
Footnotes
Funding: This work was supported by a grant from Vision 100 Foundation and a grant from Jung Foundation.
Competing interests: None declared.
References
- 1.Ambati J, Ambati B K, Yoo S H.et al Age‐related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol 200348257–293. [DOI] [PubMed] [Google Scholar]
- 2.Treatment of Age‐Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group Photodynamic therapy of subfoveal choroidal neovascularization in age‐related macular degeneration with verteporfin: one‐year results of 2 randomized clinical trials—TAP Report 1. Arch Ophthalmol 19991171329–1345. [PubMed] [Google Scholar]
- 3.Treatment of Age‐Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group Photodynamic therapy of subfoveal choroidal neovascularization in age‐related macular degeneration with verteporfin: two‐year results of 2 randomized clinical trials—TAP Report 2. Arch Ophthalmol 2001119198–207. [PubMed] [Google Scholar]
- 4.Treatment of Age‐Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group Verteporfin therapy of subfoveal choroidal neovascularization in patients with age‐related macular degeneration: additional information regarding baseline lesion composition's impact on vision outcomes—TAP report 3. Arch Ophthalmol 20021201443–1454. [DOI] [PubMed] [Google Scholar]
- 5.Verteporfin in Photodynamic Therapy Study group Verteporfin therapy of subfoveal choroidal neovascularization in age‐related macular degeneration: two‐year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization—Verteporfin in Photodynamic Therapy report 2. Am J Ophthalmol 2001131541–560. [DOI] [PubMed] [Google Scholar]
- 6.Blinder K J, Bradley S, Bressler N M.et al Treatment of Age‐Related Macular Degeneration with Photodynamic Therapy study group; Verteporfin in Photodynamic Therapy study group. Effect of lesion size, visual acuity, and lesion composition on visual acuity change with and without verteporfin therapy for choroidal neovascularization secondary to age‐related macular degeneration: TAP and VIP report no, 1. Am J Ophthalmol 2003136407–418. [DOI] [PubMed] [Google Scholar]
- 7.Hawkins B S, Bressler N M, Miskala P H.et al Submacular Surgery Trials (SST) Research Group. Surgery for subfoveal choroidal neovascularization in age‐related macular degeneration: Ophthalmic Findings SST Report No, 11. Ophthalmology 20041111967–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park C H, Toth C A. Macular translocation surgery with 360‐degree peripheral retinectomy following ocular photodynamic therapy of choroidal neovascularization. Am J Ophthalmol 2003136830–835. [DOI] [PubMed] [Google Scholar]
- 9.Mirshahi A, Schreyger F, Baatz H.et al Macular translocation after photodynamic therapy: a case report. Klin Monatsbl Augenheilkd 2005222586–589. [DOI] [PubMed] [Google Scholar]
- 10.Fujii G Y, de Juan E, Jr, Humayun M S.et al Limited macular translocation for the management of subfoveal choroidal neovascularization after photodynamic therapy. Am J Ophthalmol 2003135(109–112. [DOI] [PubMed] [Google Scholar]
- 11.Glacet‐Bernard A, Coscas G, Soubrane G. Pigment epithelial changes in young women treated with photodynamic therapy and limited macular translocation for classic choroidal neovascularisation. Graefe's Arch Clin Exp Ophthalmol 20062441373–1376. [DOI] [PubMed] [Google Scholar]
- 12.Gragoudas E S, Adamis A P, Cunningham E T, Jr, VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group. et al Pegaptanib for neovascular age‐related macular degeneration. N Engl J Med 20043512805–2816. [DOI] [PubMed] [Google Scholar]
- 13.Avery R L, Pieramici D J, Rabena M D.et al Intravitreal Bevacizumab (Avastin) for neovascular age‐related macular degeneration. Ophthalmology 2006113363–372. [DOI] [PubMed] [Google Scholar]
- 14.Campochiaro P A, Nguyen Q D, Shah S M.et al Adenoviral vector‐delivered pigment epithelium‐derived factor for neovascular age‐related macular degeneration: results of a phase I clinical trial. Hum Gene Ther 200617167–176. [DOI] [PubMed] [Google Scholar]
- 15.Kwak N, Okamoto N, Wood J.et al VEGF is major stimulator in model of choroidal neovascularization. Invest Ophthalmol Vis Sci 2000413158–3164. [PubMed] [Google Scholar]
- 16.Baffi J, Bymes G, Chan C C.et al Choroidal neovascularization in the rat induced by adenovirus mediated expression of vascular endothelial growth factor. Invest Ophthalmol Vis Sci 2000413582–3589. [PubMed] [Google Scholar]
- 17.Tatar O, Kaiserling E, Adam A.et al Consequences of Verteporfin Photodynamic Therapy on Choroidal Neovascular Membranes. Arch Ophthalmol 2006124815–823. [DOI] [PubMed] [Google Scholar]
- 18.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 199686353–364. [DOI] [PubMed] [Google Scholar]
- 19.Tatar O, Adam A, Shinoda K.et al Expression of VEGF and PEDF in choroidal neovascular membranes following verteporfin photodynamic therapy. Am J Ophthalmol 200614295–104. [DOI] [PubMed] [Google Scholar]
- 20.ÕReilly MS, Boehm T, Shing Y, et al Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 199788277–285. [DOI] [PubMed] [Google Scholar]
- 21.Tatar O, Shinoda K, Adam A.et al Expression of endostatin in human choroidal neovascular membranes secondary to age‐related macular degeneration. Exp Eye Res 200683329–338. [DOI] [PubMed] [Google Scholar]
- 22.Bhutto I A, Kim S Y, McLeod D S.et al Localization of collagen XVIII and the endostatin portion of collagen XVIII in aged human control eyes and eyes with age‐related macular degeneration. Invest Ophthalmol Vis Sci 2004451544–1552. [DOI] [PubMed] [Google Scholar]
- 23.Marneros A G, Zambarakji H, She H.et al Increased laser–induced choroidal neovascularization in mice lacking collagen XVIII/endostatin. Invest Ophthalmol Vis Sci. 2006;47: E‐Abstract, 1532
- 24.Abdollahi A, Hahnfeldt P, Maercker C.et al Endostatin's antiangiogenic signaling network. Mol Cell 200413649–663. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi K, Saishin Y, Saishin Y.et al Intraocular expression of endostatin reduces VEGF‐induced retinal vascular permeability, neovascularization, and retinal detachment. FASEB J 200317896–898. [DOI] [PubMed] [Google Scholar]
- 26.Sasano H, Suzuki T. Pathological evaluation of angiogenesis in human tumor. Biomed Pharmacother 200559(Suppl 2)S334–S336. [DOI] [PubMed] [Google Scholar]
- 27.Yasukawa T, Kimura H, Tabata Y.et al Active drug targeting with immunoconjugates to choroidal neovascularization. Curr Eye Res 200021952–961. [DOI] [PubMed] [Google Scholar]
- 28.Grossniklaus H E, Ling J X, Wallace T M.et al Macrophage and retinal pigment epithelium expression of angiogenic cytokines in choroidal neovascularization. Mol Vis 20028119–126. [PubMed] [Google Scholar]
- 29.Karak A K, Sarkar C, Chumber S.et al MIB‐1 proliferative index in parathyroid adenoma and hyperplasia. Indian J Med Res 1997105235–238. [PubMed] [Google Scholar]
- 30.Grisanti S, Tatar O, Canbek S.et al Immunohistopathologic evaluation of choroidal neovascular membranes following verteporfin‐photodynamic therapy. Am J Ophthalmol 2004137914–923. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt‐Erfurth U, Hasan T. Mechanisms of action of photodynamic therapy with verteporfin for the treatment of age‐related macular degeneration. Surv Ophthalmol 200045195–214. [DOI] [PubMed] [Google Scholar]
- 32.Petermeier K, Tatar O, Inhoffen W.et al Verteporfin photodynamic therapy induced apoptosis in choroidal neovascular membranes. Br J Ophthalmol 2006901034–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu Y, Moulton K S, Khan M K.et al E‐selectin is required for the antiangiogenic activity of endostatin. Proc Natl Acad Sci USA 20041018005–8010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yeh D C, Bula D V, Miller J W.et al Expression of leukocyte adhesion molecules in human subfoveal choroidal neovascular membranes treated with and without photodynamic therapy. Invest Ophthalmol Vis Sci 2004452368–2373. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt‐Erfurth U, Michels S. Changes in confocal indocyanine green angiography through two years after photodynamic therapy with verteporfin. Ophthalmology 20031101306–1314. [DOI] [PubMed] [Google Scholar]
- 36.Michels S, Schmidt‐Erfurth U. Sequence of early vascular events after photodynamic therapy. Invest Ophthalmol Vis Sci 2003442147–2154. [DOI] [PubMed] [Google Scholar]
- 37.Michels S, Hansmann F, Geitzenauer W.et al Influence of treatment parameters on selectivity of verteporfin therapy. Invest Ophthalmol Vis Sci 200647371–376. [DOI] [PubMed] [Google Scholar]
- 38.Schlingemann R O. Role of growth factors and the wound healing response in age‐related macular degeneration. Graefe's Arch Clin Exp Ophthalmol 200424291–101. [DOI] [PubMed] [Google Scholar]
- 39.Shima D T, Adamis A P, Ferrara N.et al Hypoxic induction of endothelial cell growth factors in retinal cells: identification and characterization of vascular endothelial growth factor (VEGF) as the mitogen. Mol Med 19951182–193. [PMC free article] [PubMed] [Google Scholar]
- 40.Shima D T, Deutsch U, D'Amore P A. Hypoxic induction of vascular endothelial growth factor (VEGF) in human epithelial cells is mediated by increases in mRNA stability. FEBS Lett 1995370203–208. [DOI] [PubMed] [Google Scholar]
- 41.Kuroki M, Voest E E, Amano S.et al Reactive oxygen intermediates increase vascular endothelial growth factor expression in vitro and in vivo. J Clin Invest 1996981667–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu P, Yonekura H, Li H.et al Hypoxia down‐regulates endostatin production by human microvascular endothelial cells and pericytes. Biochem Biophys Res Commun 20012881149–1154. [DOI] [PubMed] [Google Scholar]
- 43.Nasu K, Nishida M, Fukuda J.et al Hypoxia simultaneously inhibits endostatin production and stimulates vascular endothelial growth factor production by cultured human endometrial stromal cells. Fertil Steril 200482756–759. [DOI] [PubMed] [Google Scholar]
- 44.Dobryansky M, Galiano R D, Cetrulo C L., Jret al Endostatin inhibits ischemia‐induced neovascularization and increases ischemic tissue loss. Ann Plast Surg 200452512–518. [DOI] [PubMed] [Google Scholar]
- 45.Eriksson K, Magnusson P, Dixelius J.et al Angiostatin and endostatin inhibit endothelial cell migration in response to FGF and VEGF without interfering with specific intracellular signal transduction pathways. FEBS Lett 200353619–24. [DOI] [PubMed] [Google Scholar]
- 46.Ergun S, Kilic N, Wurmbach J H.et al Endostatin inhibits angiogenesis by stabilization of newly formed endothelial tubes. Angiogenesis 20014193–206. [DOI] [PubMed] [Google Scholar]
- 47.Hajitou A, Grignet C, Devy L.et al The antitumoral effect of endostatin and angiostatin is associated with a down‐regulation of vascular endothelial growth factor expression in tumor cells. FASEB J 2002161802–1804. [DOI] [PubMed] [Google Scholar]
- 48.Jia Y H, Dong X S, Wang X S. Effects of endostatin on expression of vascular endothelial growth factor and its receptors and neovascularization in colonic carcinoma implanted in nude mice. World J Gastroenterol 2004103361–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zatterstrom U K, Felbor U, Fukai N.et al Collagen XVIII/endostatin structure and functional role in angiogenesis. Cell Struct Funct 20002597–101. [DOI] [PubMed] [Google Scholar]
- 50.Ferreras M, Felbor U, Lenhard T.et al Generation and degradation of human endostatin proteins by various proteinases. FEBS Lett 2000486247–251. [DOI] [PubMed] [Google Scholar]
- 51.Sunderkotter C, Steinbrink K, Goebeler M.et al Macrophages and angiogenesis. J Leukoc Biol 199455410–422. [DOI] [PubMed] [Google Scholar]
- 52.Spilsbury K, Garrett K L, Shen W Y.et al Overexpression of vascular endothelial growth factor (VEGF) in the retinal pigment epithelium leads to the development of choroidal neovascularization. Am J Pathol 2000157135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Macpherson G R, Ng S S W, Forbes S L.et al Anti‐angiogenic activity of endostatin is HIF‐1‐ is independent and sensitive to timing of treatment in a human sapheneous vein assay. Mol Cancer Ther 20032845–854. [PubMed] [Google Scholar]
- 54.Fukai N, Eklund L, Marneros A G.et al Lack of collagen XVIII/endostatin results in eye abnormalities. EMBO J 2002211535–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mori K, Ando A, Gehlbach P.et al Inhibition of choroidal neovascularization by intravenous injection of adenoviral vectors expressing secretable endostatin. Am J Pathol 2001159313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shang Q L, Ma J X, Wei J S.et al Experimental choroidal neovascularization is inhibited by subretinal administration of Endostatin. Zhonghua Yan Ke Za Zhi 200440266–271. [PubMed] [Google Scholar]
- 57.Balaggan K S, Binley K, Esapa M.et al EIAV vector‐mediated delivery of endostatin or angiostatin inhibits angiogenesis and vascular hyperpermeability in experimental CNV. Gene Therapy 2006131153–1165. [DOI] [PubMed] [Google Scholar]
- 58.Folkman J. Endogeneous angiogenesis inhibitors. APMIS 2004112496–507. [DOI] [PubMed] [Google Scholar]
- 59.Abdollahi A, Lipson K E, Sckell A.et al Combined therapy with direct and indirect angiogenesis inhibition results in enhanced antiangiogenic and antitumor effects. Cancer Res 2003638890–8898. [PubMed] [Google Scholar]
- 60.Pan X, Wang Y, Zhang M.et al Effects of endostatin‐vascular endothelial growth inhibitor chimeric recombinant adenoviruses on antiangiogenesis. World J Gastroenterol 2004101409–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]