Abstract
Objective
To compare the safety and efficacy of different doses of intravitreal triamcinolone (ivTA) in treating clinically significant diabetic macular oedema (CSMO).
Methods
63 eyes of 63 patients with CSMO and central foveal thickness (CFT) of ⩾250 μm on optical coherence tomography were randomised to receive 4 mg (n = 23), 6 mg (n = 20) or 8 mg (n = 20) ivTA. Patients were followed up for 6 months, and changes in best‐corrected visual acuity (BCVA), optical coherence tomography CFT, standardised change in macular thickness (SCMT), and side effects such as intraocular pressure and cataractogenesis were compared between the three groups.
Results
After ivTA injection, improvements of BCVA and CFT occurred in all groups. The mean BCVA improvement at 6 months was significantly higher for the 8 mg group compared with the 4 mg group, with 9.9 and 3.1 improvement in letters on the Early Treatment of Diabetic Retinopathy Study chart, respectively (p = 0.047). The mean SCMT at 6 months for the 4, 6 and 8 mg groups was 28.7%, 42.3% and 60.5%, respectively (p = 0.06). The proportion of eyes with SCMT ⩾75% at 6 months was higher in the 8 mg group, but the difference failed to reach significance (p = 0.06). Ocular hypertensive responses (>21 mm Hg) occurred in 39%, 30% and 55% of eyes in the 4, 6, and 8 mg groups, respectively (p = 0.27).
Conclusions
Higher doses of ivTA may prolong the duration of visual benefit in diabetic CSMO and seemed to result in more sustained reduction in macular oedema. Further studies are warranted to investigate the optimum dose of ivTA in treating diabetic CSMO.
Intravitreal triamcinolone (ivTA) has been used as a primary or adjunctive treatment option for diffuse clinically significant macular oedema (CSMO) in diabetes mellitus.1,2,3,4,5,6,7,8 Most authors used 4 mg ivTA for treating diabetic CSMO, but others have used higher dosages of up to 25 mg.7,8,9,10 On the basis of previous studies,4,5 4 mg ivTA is effective in reducing macular thickening and can improve visual acuity in the short term. However, CSMO does not resolve completely in many cases and may recur 2–3 months after injection. A higher dose of ivTA may achieve further and more sustained visual improvement and resolution of oedema. On the other hand, a higher dose may also result in a higher incidence of ivTA‐associated complications, such as raised intraocular pressure (IOP) or cataractogenesis. In view of the potential benefits of using a higher dose of ivTA, we evaluated the effects of different dosages of ivTA for treating diabetic CSMO.
Patients and methods
This was a prospective randomised trial in which patients >18 years of age with diabetic CSMO according to Early Treatment of Diabetic Retinopathy Study (ETDRS) criteria,11 and central foveal thickness (CFT) of >250 μm on optical coherence tomography (OCT) were recruited from December 2003.11 Patients with or without previous macular laser photocoagulation were recruited at the Hong Kong Eye Hospital and the United Christian Hospital, Kowloon, Hong Kong. The exclusion criteria included previous ocular trauma or surgery except cataract extraction, cataract surgery within 6 months or laser within 3 months before recruitment, presence of considerable media opacity precluding fundus examination, proliferative diabetic retinopathy, history of glaucoma, and vitreomacular traction or epiretinal membrane on OCT. The protocol was approved by the ethics committee of the Chinese University of Hong Kong, and informed consent was obtained from all patients.
At baseline and follow‐up examinations, all patients underwent ophthalmic examinations including ETDRS best‐corrected visual acuity (BCVA) testing, slit‐lamp examination, and fundus examination using indirect ophthalmoscopy and slit‐lamp biomicroscopy. IOP was measured with a non‐contact tonometer (Xpert NCT Plus, Reichert Ophthalmic Instruments, Depew, New York, USA), taken as the mean of three readings. If the IOP was >20 mm Hg, it was verified with a Goldman applanation tonometer (Haag‐Streit, Koeniz, Switzerland). IOP measurements were performed between 2 and 5 pm. Cataract grading was performed using the Lens Opacity Classification System III,12 and a composite score was calculated by adding the scores in the four categories, ranging from 0 to 22. OCT was performed using StratusOCT (Carl Zeiss, Dublin, USA) with the fast macular thickness map mode, and CFT was obtained from the retinal map analysis function. BCVA and OCT measurements were performed by technicians unaware of the dose of ivTA given. Patients were randomised to receive 4, 6 or 8 mg of ivTA using a computer‐generated randomisation table.
Intravitreal injection procedure
Injections were given with strict aseptic techniques as an outpatient procedure. Local anaesthesia was achieved using 2% lidocaine hydrochloride gel (Xylocaine, AstraZenec). Povidone iodine (5%) was applied to the eyelids and instilled in the cul de sac and left for 5 min. This was followed by injection of triamcinolone acetonide (4 mg in 0.1 ml, 6 mg in 0.15 ml or 8 mg in 0.2 ml of Kenacort A 40 mg/ml (Bristol‐Myers Squibb, Anagni, Italy)) using a 27‐gauge needle at 4 mm post limbus or 3.5 mm in pseudophakic eyes. Indirect ophthalmoscopy and IOP measurement were performed after the procedure to ensure absence of complications. If the globe was felt to be tense after ivTA injection, anterior chamber paracentesis was performed at the investigator's discretion. Gutt levofloxacin 0.5% (Cravit, Santen, Japan) was instilled immediately and then four times a day for 2 weeks.
Patients were examined on day 1 and weeks 1, 2, 4, 9, 17 and 26 with BCVA testing; ophthalmic examination and OCT CFT measurements were also performed (except day 1 and week 1). Patients were monitored for potential side effects during each visit. Patients with an IOP >21 mm Hg were treated with topical drugs for glaucoma to achieve an IOP <21 mm Hg. Drugs for treatment of glaucoma were tapered gradually if the IOP remained <15 mm Hg and if there was no longer any visible triamcinolone in the vitreous.
Data analysis
The primary outcome measure was the change in BCVA, measured noted as the number of letters that the patient was able to read on the ETDRS logarithm of minimum angle of resolution (logMar) chart.13 Secondary outcome measures included reduction in CFT, standardised change in macular thickening (SCMT) measured on OCT, and ocular side effects including increases in IOP and cataractogenesis. SCMT was calculated using the formula by Chan and Duker14:
SCMT = [baseline CFT−final CFT]/[baseline CFT−normal CFT]
Normal CFT is taken as 182 μm for Stratus OCT.6 However, for some patients, the CFT dropped below 182 μm after ivTA, presumably due to retinal atrophy secondary to chronic macular oedema; in that case the minimum CFT achieved during the 6‐month period was used as the “normal” CFT for the particular patient.
Statistical analysis was performed using SPSS for Windows V.11.5. Comparison of continuous data between the dosage groups was performed with one‐way analysis of variance with the Bonferroni test or two‐tailed t test. Comparison of categorical data was performed with χ2 test or Kruskal–Wallis test. A p value ⩽0.05 was considered significant.
Sample size estimation
No data are available from the existing literature on the expected BCVA changes for patients with different doses (4, 6 or 8 mg) of ivTA. The expected difference in BCVA at 6 months after injection between the different dosage groups is approximately 20%, with a standard deviation (SD) of 20%. For α = 0.05 and power = 80%, the minimum sample size required is 16 cases per group.
Results
A total of 63 eyes of 63 Chinese patients fulfilled the inclusion criteria and the patients were recruited (table 1). All patients completed follow‐up at 6 months. There were no significant differences in baseline age, IOP, CFT, sex, proportion of pseudophakic eyes and proportion of eyes with previous photocoagulation between the three groups (p>0.05). However, there were significant differences in baseline BCVA between the three groups (p = 0.003), and post‐hoc comparison showed that the baseline BCVA in the 6 mg group was significantly worse than that in the 4 and 8 mg groups (p = 0.009 and 0.007, respectively). In view of the significantly poorer baseline BCVA in the 6 mg ivTA group, this group was excluded from the comparison of BCVA. In all, 47 eyes were phakic at baseline and none underwent cataract extraction during the 6‐month follow‐up period. None of the eyes received supplementary treatment for CSMO during the study period.
Table 1 Baseline characteristics of 63 patients in the study.
| Dose of ivTA | 4 mg | 6 mg | 8 mg | p Value |
|---|---|---|---|---|
| Total number of patients | 23 | 20 | 20 | |
| Male:female ratio | 16:7 | 12:8 | 11:9 | 0.60 |
| Those with/without previous macular photocoagulation | 16/7 | 13/7 | 13/7 | 0.93 |
| No of phakic/pseudophakic eyes | 19/4 | 16/4 | 12/8 | 0.19 |
| Mean (SD) age (years) | 64.8 (7.3) | 64.4 (8.3) | 66.0 (7.9) | 0.79 |
| Mean (SD) baseline CFT (μm) | 430 (93) | 461 (92) | 473 (142) | 0.43 |
| Mean (SD) BCVA (ETDRS logMar units) (approx. Snellen equivalent) | 0.81 (0.26) (20/130) | 1.11 (0.39) (20/260) | 0.79 (0.30) (20/125) | 0.003 |
| Mean (SD) baseline intraocular pressure (mm Hg) | 13.9 (4.2) | 13.8 (2.9) | 13.7 (2.6) | 0.99 |
BCVA, best‐corrected visual acuity; CFT, central foveal thickness; ETDRS, Early Treatment of Diabetic Retinopathy Study; ivTA, intravitreal triamcinolone; logMar, logarithm of minimum angle of resolution.
Effect on BCVA
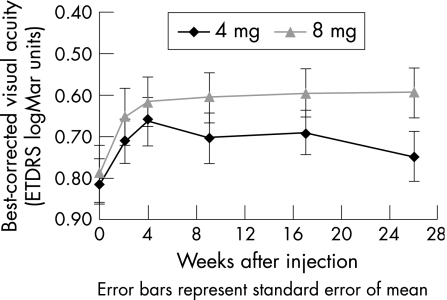
Figure 1 shows the general patterns of BCVA improvement and table 2 displays the values. For the 4 mg group, the maximum visual improvement was noted at 4 weeks after injection, with a logMar BCVA of 0.66 and an improvement of 7.3 ETDRS letters, whereas for the 8 mg group, the maximum improvement occurred at 26 weeks with a logMar BCVA of 0.59 and an improvement of 9.9 ETDRS letters. The mean BCVA improvement at 26 weeks was significantly higher in the 8 mg than in the 4 mg group (p = 0.047). In all, 6 (30%) of the 20 eyes in the 8 mg group had a visual improvement of ⩾3 lines (⩾15 ETDRS letters), compared with 3 (13%) of the 23 eyes in the 4 mg group. However, the difference was not significant (p = 0.26).
Figure 1 Mean change in best‐corrected visual acuity from baseline to 6 months for the 4 mg and 8 mg intravitreal triamcinolone groups. ETDRS, Early Treatment of Diabetic Retinopathy Study; logMar, logarithm of minimum angle of resolution.
Table 2 Summary of changes in best‐corrected visual acuity.
| Mean value for each dosage group | 4 mg (n = 23) | SD | 8 mg (n = 20) | SD | p Value |
|---|---|---|---|---|---|
| Baseline BCVA (ETDRS logMar units) (approx Snellen equivalent) | 0.81 (20/130) | 0.26 | 0.79 (20/125) | 0.3 | 0.81 |
| BCVA at 2 weeks | 0.71 | 0.26 | 0.65 | 0.31 | 0.49 |
| BCVA at 4 weeks | 0.66 | 0.28 | 0.62 | 0.26 | 0.57 |
| BCVA at 9 weeks | 0.70 | 0.28 | 0.61 | 0.27 | 0.26 |
| BCVA at 17 weeks | 0.69 | 0.26 | 0.60 | 0.26 | 0.23 |
| BCVA at 26 weeks (Approx Snellen equivalent) | 0.75 (20/110) | 0.29 | 0.59 (20/80) | 0.27 | 0.078 |
| BCVA improvement at 2 weeks (number of ETDRS letters) | 4.9 | 8.7 | 6.8 | 9.4 | 0.5 |
| BCVA improvement at 4 weeks | 7.3* | 10.3 | 8.7 | 10.5 | 0.67 |
| BCVA improvement at 9 weeks | 5.4 | 8.3 | 9.2 | 10.5 | 0.19 |
| BCVA improvement at 17 weeks | 6.0 | 9.3 | 9.7 | 10.4 | 0.22 |
| BCVA improvement at 26 weeks | 3.1 | 10 | 9.9* | 11.7 | 0.047 |
| Number (proportion) of eyes with BCVA improvement of ⩾15 ETDRS letters at 6 months | 3 (13%) | 6 (30%) | 0.26 | ||
| Number (proportion) of eyes with BCVA deterioration of ⩾15 ETDRS letters at 6 months | 2 (8%) | 1 (5%) | 1.0 | ||
BCVA, best‐corrected visual acuity; ETDRS, Early Treatment of Diabetic Retinopathy Study; logMar, logarithm of minimum angle of reolution.
*Best BCVA improvement within group.
Effect on CFT
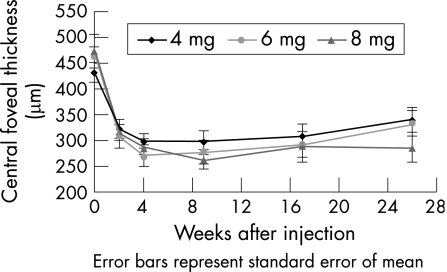
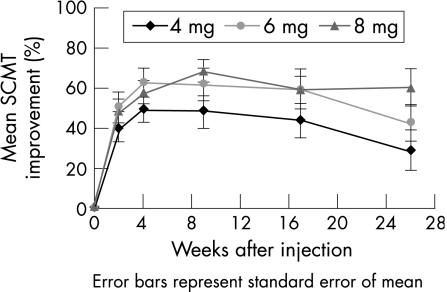
After ivTA injection, the CFT reduced in all three group, reaching a trough at 4–9 weeks, after which there was recurrence of oedema, especially in the 4 and 6 mg groups. Figures 2 and 3 show the pattern of changes in CFT and SCMT respectively, and table 3 displays the values. The maximum mean (SD) SCMT during the follow‐up period was 49.2 (29.5%) in the 4 mg group (4 weeks), 62.8 (29.8%) in the 6 mg group (4 weeks) and 68.2 (25.5%) in the 8 mg group (9 weeks). By 6 months, macular thickening had partially recurred in all three groups. The mean (SD) SCMT at 6 months was 28.7 (49.1%), 42.3 (38.9%) and 60.5 (41.1%) in the 4, 6 and 8 mg groups, respectively. The mean CFT at all time points was not significantly different between the three dosage groups. For the mean SCMT, the percentage change was higher in the 8 mg group than in the 4 and 6 mg groups at 6 months, although this marginally failed to reach the level of significance (p = 0.06). The proportion of eyes with SCMT ⩾75% at 6 months was also higher in the 8 mg (50%) group than in the 4 mg (17%) and 6 mg (25%) groups. However, again, the difference just failed to reach significance (p = 0.06). There were more eyes with best SCMT ⩾75% in the 6 and 8 mg groups compared with the 4 mg group, but the differences were not significant (p = 0.22).
Figure 2 Change in mean central foveal thickness from baseline to 6 months for the 4, 6 and 8 mg intravitreal triamcinolone groups.
Figure 3 Improvement of mean standardised change in macular thickness (SCMT) from baseline to 6 months for the 4, 6 and 8 mg intravitreal triamcinolone groups.
Table 3 Summary of changes in central foveal thickness.
| Mean value for each dosage group | 4 mg (n = 23) | SD | 6 mg (n = 20) | SD | 8 mg (n = 20) | SD | p Value |
|---|---|---|---|---|---|---|---|
| Baseline CFT (μm) | 430 | 93 | 461 | 92 | 473 | 142 | 0.43 |
| CFT (μm) at 2 weeks | 321 | 84 | 307 | 102 | 314 | 69 | 0.86 |
| CFT (μm) at 4 weeks | 298* | 66 | 270* | 93 | 287 | 62 | 0.47 |
| CFT (μm) at 9 weeks | 298 | 86 | 276 | 107 | 260* | 59 | 0.34 |
| CFT (μm) at 17 weeks | 309 | 98 | 291 | 106 | 288 | 129 | 0.80 |
| CFT (μm) at 26 weeks | 339 | 112 | 332 | 118 | 284 | 113 | 0.25 |
| SCMT (%) at 2 weeks | 40.3 | 34.9 | 50.6 | 34.6 | 48.6 | 27.3 | 0.55 |
| SCMT (%) at 4 weeks | 49.2* | 29.5 | 62.8* | 29.8 | 57.8 | 26.1 | 0.29 |
| SCMT (%) at 9 weeks | 48.0 | 37.3 | 61.4 | 36.4 | 68.2* | 25.5 | 0.14 |
| SCMT (%) at 17 weeks | 43.9 | 40.1 | 59.2 | 30.3 | 59.1 | 44.0 | 0.33 |
| SCMT (%) at 26 weeks | 28.7 | 49.1 | 42.3 | 38.9 | 60.5 | 41.1 | 0.06 |
| Proportion of eyes with best SCMT ⩾75%, n (%) | 11 (48) | 14 (70) | 14 (70) | 0.22 | |||
| Proportion of eyes with SCMT ⩾75% at 26 weeks, n(%) | 4 (17) | 5 (25) | 10 (50) | 0.06 | |||
CFT, central foveal thickness; SCMT, standard change in macular thickness.
*Best CFT/SCMT within dosage group.
Side effects
Table 4 summarises the ocular side effects. No significant differences were observed between the three groups with eyes with maximum IOP increased >21 mm Hg (p = 0.27). Nonetheless, the proportion of eyes remaining on treatment for glaucoma at 6 months was higher in the 8 mg group than in the 4 and 6 mg groups (p = 0.05). The mean time to IOP rise ranged from 8.7 to 11.5 weeks after ivTA injection. In all patients, the IOP was controlled only with topical drugs for glaucoma and none required filtration surgery. In terms of increased cataractogenesis, there was no significant difference between the three groups with regard to the proportion of eyes with increased cataract grading and the average increase in grading (p = 0.31 and 0.53, respectively). None of the eyes developed severe ocular complications due to infective endophthalmitis or retinal detachment.
Table 4 Ocular side effects noted in the three treatment groups.
| Dose of ivTA | 4 mg(n = 23) | 6 mg(n = 20) | 8 mg(n = 20) | p Value |
|---|---|---|---|---|
| Eyes with maximum IOP >21 mm Hg,n (%) | 9 (39%) | 6 (30%) | 11 (55%) | 0.27 |
| Mean (range) time to IOP rise (weeks) | 9 (4–17) | 8.7 (2–26) | 11.5 (4–26) | |
| Mean number of drugs for glaucoma per eye with IOP >21 mm Hg | 1.8 | 1.8 | 2.5 | 0.16 |
| Eyes still receiving drugs for glaucoma at 6 months, n | 4 | 3 | 9 | 0.05 |
| Phakic eyes, n | 19 | 16 | 12 | |
| Phakic eyes with increase in cataract grade, n (%) | 6/19 (32%) | 7/16 (44%) | 2/12 (17%) | 0.31 |
| Average (SD) increase of cataract grading (LOCS3 chart) per phakic patient | 1.1 (2.1) | 0.8 (1.2) | 0.7 (1.6) | 0.53 |
| Other miscellaneous | Mild vitreous haemorrhage 1/23 (4.3%) | Nil | Transient vitritis* 1/20 (5%) |
IOP, intraocular pressure; ivTA, intravitreal triamcinolone; LOCS3, Lens Opacity Classification System III.
*Transient vitritis developed after ivTA and resolved without sequelae by week 9.
Discussion
A recent study by Spandau et al15 compared the effects of 2, 5 and 13 mg ivTA in patients with diffuse diabetic macular oedema. Results showed that increased dosage of ivTA resulted in more prolonged and more pronounced visual improvement and treatment response. However, the study did not report changes in macular thickness, as OCT was not performed and the common dosage of 4 mg ivTA was not used. Moreover, Spandau et al also used a sedimentation method to prepare the ivTA,16 which has not been commonly adopted by other groups. In our study, using triamcinolone directly from the vial, we chose the dosages from 4 mg (0.1 ml) to 8 mg (0.2 ml). The dosages of 6 and 8 mg used in our study also filled in the gap in the study by Spandau et al.
In our study, we excluded the 6 mg group, owing to a significantly poorer mean BCVA at baseline. Although there were no differences between the BCVA of the 4 and 8 mg groups at any time points, the number of ETDRS letters gained was significantly better in the 8 mg group compared with the 4 mg group at 26 weeks. The visual improvement in the 4 mg group began to disappear after 4 weeks, whereas the BCVA of the 8 mg group continued to improve until 26 weeks, indicating a prolonged treatment effect with higher doses of ivTA. These findings are consistent with the findings by Spandau et al,15 in which a higher dose of ivTA resulted in more prolonged visual improvement. In terms of the proportion of patients with a BCVA gain of at least 15 ETDRS letters at 6 months, there was also a trend towards greater benefit with 8 mg ivTA, although it failed to reach statistical significance.
In our study, we found that the 8 mg group had higher SCMT compared with the 4 and 6 mg groups at 26 weeks, although the difference just failed to reach statistical significance. There was also a trend towards a higher proportion of eyes with SCMT ⩾75% in the 8 mg group compared with the 4 and 6 mg groups. These results suggest that a higher dose of ivTA is associated with a more sustained effect and a larger reduction in macular thickness. Although one might expect improvement in macular oedema together with visual improvement, we found that reduction in macular thickness occurs much earlier than visual improvement. Larsson et al17 suggested that visual improvement may be related to the duration of the oedema, and thus caused this time lag between the reduction in macular thickness and improvement in vision. Longer duration of macular oedema may also be associated with irreversible damage to the photoreceptors and may limit the potential beneficial effect of reduction in central macular thickness on visual acuity. This may account for the wide variability in the duration of beneficial effect of intravitreal triamcinolone.
In terms of side effects, no cases of infective endophthalmitis were observed in our study. The proportion of eyes with raised IOP was greater in the 8 mg group than in the 4 and 6 mg groups, but the difference was not statistically significant. Despite the lack of difference, the number of eyes remaining on drugs for glaucoma at 6 months was significantly higher in the 8 mg group (p = 0.05). In our study, 55% of eyes in the 8 mg group showed IOP >21 mm Hg. One of the reasons that the number of eyes receiving drugs for glaucoma was higher in the 8 mg group might be because of the longer duration of side effects for the high‐dosage group. Although we excluded patients with a history of glaucoma, the percentage of patients with raised IOP in our study was higher than those reported by Ozkiris et al7 and Jonas et al,18 which ranged from 16.6% to 41.2%. This might be because of a racial difference in the ocular hypertensive response between Chinese and Caucasian subjects. Although our previous study using 4 mg ivTA did not show the incidence of steroid‐induced raised IOP in Chinese to be different from that in Caucasians,19 further studies are required to clarify the issue of steroid response better.
There are several limitations to our study. Firstly, despite the randomised nature of the treatment intervention in our study, the 6 mg group had significantly worse baseline BCVA compared with the 4 and 8 mg groups. In view of the potential bias, we therefore excluded the 6 mg group from the BCVA comparison. For further studies, this limitation can be dealt with by using a stratified randomisation method. Another limitation of the study was the lack of fluorescein angiographic documentation. Although CSMO is a clinical diagnosis, fluorescein angiography would be beneficial in the diagnosis of macular ischaemia. Finally, another shortcoming was the relatively small number of patients and the short follow‐up duration. During the 6 month follow‐up period, we did not observe a deterioration of CFT or BCVA back to the baseline level, and therefore the treatment response duration could not be determined. As a substantial proportion of patients were still receiving treatment for glaucoma at 6 months, it would be beneficial to determine whether the IOP of these patients will return to baseline without treatment in due course.
In conclusion, our study showed that higher doses of ivTA may prolong the visual benefit and seemed to result in better reduction of macular thickness in the treatment of diabetic CSMO. However using higher doses of ivTA requires caution because of a higher magnitude of steroid‐induced ocular hypertensive response. Larger‐scale studies and longer‐term results will help to determine the optimal choice of ivTA dosage for treating patients with diabetic CSMO.
Abbreviations
BCVA - best‐corrected visual acuity
CFT - central foveal thickness
CSMO - clinically significant macular oedema
ETDRS - Early Treatment of Diabetic Retinopathy Study
IOP - intraocular pressure
ivTA - intravitreal triamcinolone
logMar - logarithm of minimum angle of resolution
OCT - optical coherence tomography
SCMT - standardised change in macular thickness
Footnotes
Funding: This work was supported in part by the Action for Vision Eye Foundation, Hong Kong (a charitable organisation). The funding organisation did not contribute to the design/execution/data analysis of this study.
Competing interests: None.
The data in this paper were presented in part at the 2nd International Symposium on Macular Diseases (16–18 December 2004, Bangkok); 2nd SERI ARVO Meeting on Research in Vision and Ophthalmology (16–20 February 2005, Singapore) and the 20th Asia Pacific Academy of Ophthalmology Congress (27–31 March 2005, Kuala Lumpur).
References
- 1.Bakri S J, Beer P M. Intravitreal triamcinolone injection for diabetic macular oedema: a clinical and fluorescein angiographic case series. Can J Ophthalmol 200439755–760. [DOI] [PubMed] [Google Scholar]
- 2.Jonas J B, Kreissig I, Sofker A.et al Intravitreal injection of triamcinolone for diffuse diabetic macular oedema. Arch Ophthalmol 200312157–61. [PubMed] [Google Scholar]
- 3.Jonas J B, Akkoyun I, Kreissig I.et al Diffuse diabetic macular oedema treated by intravitreal triamcinolone acetonide: a comparative, non‐randomised study. Br J Ophthalmol 200589321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lam D S, Chan C K, Mohamed S.et al Phacoemulsification with intravitreal triamcinolone in patients with cataract and coexisting diabetic macular oedema: a 6‐month prospective pilot study. Eye 200519885–890. [DOI] [PubMed] [Google Scholar]
- 5.Lam D S, Chan C K, Tang E W.et al Intravitreal triamcinolone for diabetic macular oedema in Chinese patients: six‐month prospective longitudinal pilot study. Clin Exp Ophthalmol 200432569–572. [DOI] [PubMed] [Google Scholar]
- 6.Martidis A, Duker J S, Greenberg P B.et al Intravitreal triamcinolone for refractory diabetic macular oedema. Ophthalmology 2002109920–927. [DOI] [PubMed] [Google Scholar]
- 7.Ozkiris A, Evereklioglu C, Erkilic K.et al Intravitreal triamcinolone acetonide injection as primary treatment for diabetic macular oedema. Eur J Ophthalmol 200414543–549. [PubMed] [Google Scholar]
- 8.Sutter F K, Simpson J M, Gillies M C. Intravitreal triamcinolone for diabetic macular oedema that persists after laser treatment: three‐month efficacy and safety results of a prospective, randomized, double‐masked, placebo‐controlled clinical trial. Ophthalmology 20041112044–2049. [DOI] [PubMed] [Google Scholar]
- 9.Jonas J B, Degenring R, Kreissig I.et al Safety of intravitreal high‐dose reinjections of triamcinolone acetonide. Am J Ophthalmol 20041381054–1055. [DOI] [PubMed] [Google Scholar]
- 10.Ozkiris A, Evereklioglu C, Erkilic K.et al Intravitreal triamcinolone acetonide for treatment of persistent macular oedema in branch retinal vein occlusion. Eye 20062013–17. [DOI] [PubMed] [Google Scholar]
- 11.Early Treatment Diabetic Retinopathy Study Research Group Photocoagulation for diabetic macular oedema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol 19851031796–1806. [PubMed] [Google Scholar]
- 12.Chylack L T, Jr, Wolfe J K, Singer D M.et al The Lens Opacities Classification System III. The Longitudinal Study of Cataract Study Group. Arch Ophthalmol 1993111831–836. [DOI] [PubMed] [Google Scholar]
- 13.Early Treatment Diabetic Retinopathy Study Research Group Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Ophthalmology 199198766–785. [PubMed] [Google Scholar]
- 14.Chan A, Duker J S. A standardized method for reporting changes in macular thickening using optical coherence tomography. Arch Ophthalmol 2005123939–943. [DOI] [PubMed] [Google Scholar]
- 15.Spandau U H, Derse M, Schmitz‐Valckenberg P.et al Dosage dependency of intravitreal triamcinolone acetonide as treatment for diabetic macular oedema. Br J Ophthalmol 200589999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jonas J B, Hayler J K, Panda‐Jonas S. Intravitreal injection of crystalline cortisone as adjunctive treatment of proliferative vitreoretinopathy. Br J Ophthalmol 2000841064–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larsson J, Zhu M, Sutter F.et al Relation between reduction of foveal thickness and visual acuity in diabetic macular oedema treated with intravitreal triamcinolone. Am J Ophthalmol 2005139802–806. [DOI] [PubMed] [Google Scholar]
- 18.Jonas J B, Degenring R F, Kreissig I.et al Intraocular pressure elevation after intravitreal triamcinolone acetonide injection. Ophthalmology 2005112593–598. [DOI] [PubMed] [Google Scholar]
- 19.Chan C K, Fan D S, Chan W M.et al Ocular‐hypertensive response and corneal endothelial changes after intravitreal triamcinolone injections in Chinese subjects: a 6‐month follow‐up study. Eye 200519625–630. [DOI] [PubMed] [Google Scholar]