Abstract
Aims
To determine the inflammatory response in retina and epiretinal membranes after intraocular silicone oil tamponade.
Methods
14 proliferative vitreoretinopathy (PVR) epiretinal membranes, 33 retro‐oil epiretinal membranes, 19 retinectomies, 14 retro‐oil retinectomies and 37 idiopathic epiretinal membranes (controls) underwent immunohistochemical analysis using the avidin–biotin complex technique and a panel of monoclonal and polyclonal antibodies. The number of positive cells counted in five 0.5 mm diameter fields of immunohistochemical sections was graded on a score of 1–4.
Results
Macrophage cell counts were significantly greater in membranes with a history of exposure to silicone oil (p<0.001). An inflammatory response could be observed within 1 month of silicone oil exchange, and the intensity seemed to be unrelated to the duration of exposure. Macrophages were confined to epiretinal membranes on the surface of retinectomy specimens in 10 of 14 cases and intraretinal macrophages were observed only in specimens with gliotic retina. T and B lymphocytes were rarely seen in the specimens examined. Marked glial cell up regulation was observed in 11 of 16 retinectomy specimens and in 8 of 11 retro‐oil retinectomies. Glial cell content was variable in the membranes, but there was a trend of increased presence after exposure to silicone oil.
Conclusion
This study has shown that the use of silicone oil is accompanied by an inflammatory reaction, primarily mediated by bloodborne macrophages. This response can be observed within 1 month of silicone oil injection and continues after silicone oil removal. Retinal surgeons should be aware of the potential secondary effects of intraocular silicone oil when they are considering its use (and removal) in vitreoretinal surgery.
Since the introduction of silicone oil to retinal surgery in 1962 by Cibis et al1 there have been controversies over its role and potential toxicity. The clinical use of silicone oil in vitreoretinal surgery has been tempered by its documented complications, which include cataract, band keratopathy, secondary glaucoma and potentially reduced visual acuity.2,3,4,5,6
Sequestration of silicone oil in the retina and posterior migration in the optic nerve has been observed in a number of case series.7,8,9,10,11,12,13,14,15 The presence of an associated inflammatory response has also been documented, characterised by the presence of macrophages and giant cells laden with lipid vacuoles.14,15,16,17,18,19 The presence of silicone oil in ocular tissue combined with an inflammatory response has been proposed as a potential mechanism for the clinical complications observed after exposure to silicone oil.
One of the indications for silicone oil use is retinal detachment complicated by proliferative vitreoretinopathy (PVR), and an inflammatory response has also been observed in patients with PVR in both epiretinal membranes (ERM) and subretinal membranes.19,20,21 The presence of T lymphocytes, macrophages, immunoglobulins and complement within PVR tissue has been documented22,23,24,25; however, a comparison of the inflammatory response observed in PVR with that seen after silicone oil tamponade has not yet been reported.
An immunopathological analysis of ocular tissues exposed to silicone oil in comparison to tissues with similar pathology where silicone was not used may help to define the intraocular pathology, that is attributable to silicone oil use. This study was undertaken to characterise the retinal and epiretinal immunopathology after exposure to silicone oil using specimens of retina (retinectomies) and epiretinal membranes.
Methods
This study was granted ethical approval by the Moorfields local research ethics committee (CHAD 1009).
Specimens
The following intraocular specimens were collected from eyes undergoing vitreoretinal surgery: 14 PVR ERMs, 19 PVR retinectomy specimens, 33 retrosilicone oil PVR ERMs and 14 retrosilicone oil PVR retinectomy specimens. Medical grade silicone oil was routinely used at the time this study was undertaken, specific data on the type of silicone oil used were not collected. Control specimens comprised epiretinal membranes in patients with no history of previous retinal detachment surgery (n = 37). Of these 37 patients, 12 underwent surgery for macular pucker, 24 for idiopathic epiretinal membranes and 1 for an epiretinal membrane which formed after macular hole surgery. Table 1 presents the clinical characteristics of each group.
Table 1 Proliferative vitreoretinopathy epiretinal membranes, retinectomies and controls: clinical features of retinal detachments.
| Specimen | Control tissue | PVR epiretinal membranes | PVR epiretinal membranes/Oil | Retinectomy | Retinectomy/oil |
|---|---|---|---|---|---|
| Total number collected | 37 | 14 | 33 | 19 | 14 |
| Clinical data available | 25 | 11 | 24 | 16 | 13 |
| Median (range) length of detached retina (days) | NA | 25 (14–141) | 43 (2–184) | 70 (8–977) | 59 (21–412) |
| Median (range) length of oil tamponade at time sample taken (days) | NA | NA | 151 (18–413) | NA | 106 (42–426) |
| Number with oil in situ at time of sampling | NA | NA | 18 | NA | 12 |
NA, not applicable; PVR, proliferative vitreoretinopathy.
Processing/staining
Specimens were fixed in 4% paraformaldehyde and processed into paraffin wax using xylene as the antemedium. Tissue sections were stained with haematoxylin and eosin to assess general morphology. The immunohistochemical distribution of CD45RO (UCHL1) and CD45 (leucocyte common antigen) for T lymphocytes, CD20 (L26) for B lymphocytes, Mac 387 and CD68 (PGM1) for macrophages, Cam 5.2 for retinal pigment epithelium (RPE) cells and glial fibrillary acidic protein for glial tissue was studied using a conventional alkaline phosphatase avidin–biotin complex method. The antigens were visualised as the final red reaction product of Vector red (Vector Laboratories, Peterborough, UK). Appropriate negative (using non‐immune serum from the same species as the primary antibody and at the same protein concentration) and positive (using tissues known to express the antigen) controls were analysed. Table 2 outlines the primary antibodies used, together with information on antigen retrieval.
Table 2 Antigens studied.
| Antigen | Target | Antibody source | Antigen retrieval | Antibody dilution |
|---|---|---|---|---|
| CD45RO | T lymphocytes | Dako | Heat mediated | 1:800 |
| CD45 | T lymphocytes | Dako | Heat mediated | 1:800 |
| CD20cy | B lymphocytes | Dako | Heat mediated | 1:600 |
| Mac 387 | Macrophages | Dako | Trypsin | 1:100 |
| CD68 | Macrophages | Dako | Trypsin | 1:100 |
| Cam 5.2 | RPE cells | BD Biosciences | Trypsin | 1:50 |
| GFAP | Glial tissue | Dako | Trypsin | 1:2000 |
GFAP, glial fibrillary acidic protein; RPE, retinal pigment epithelium.
Antigen retrieval‐tissue sections were treated by either:(1) heat mediation, performed in an 800 W microwave oven by heating in 50 g/l urea in 50 mM TRIS‐HCl buffer pH 9.5 for 10 min followed by cooling for a further 25 min Or (2) trypsin, performed in a 37°C incubator by exposure to 1 g/l trypsin in 100 mm TRIS‐HCl buffer pH 7.8 for 15 min.
Dako, Ely, UK; BD Biosciences New Jersey, USA.
A semiquantitative analysis of the cellular response in the tissue samples was undertaken. The number of positive cells counted in five high‐power fields of immunohistochemical sections was graded on a score of 1–4, where grade 1 represented an average of 1 cell/HPF, grade 2, an average of 2 cells HPF and so on. One high‐power field measured 0.5 mm in diameter. All observations were carried out by two observers blinded to the case histories of the patients. The interobserver agreement was 80% exact agreement, with 1% differing by >1 grade. Differences in grading were arbitrated by a third observer.
A χ2 test was used to assess the significance of observed differences in macrophage counts and glial up regulation in the different groups.
Results
PVR epiretinal membranes
With silicone oil
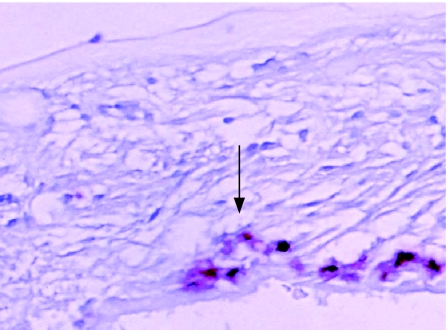
In all, 25 of the 33 ERM oil specimens had a dense infiltration of macrophages (grade 3 or 4; fig 1; table 3). The macrophage response in the silicone oil membranes was significantly greater than that in the non‐oil specimens (p<0.001, χ2 test). Phagocytosis of oil in macrophages, observed as optically empty intracellular vacuoles between 1 and 16 μm in diameter, were found in the ERM oil group and were not seen in any of the control specimens. The presence of multinucleated giant cells within this group was also noted (fig 2). Four of the six specimens with lower cell counts (1–2) for macrophage markers had no silicone oil visible in the area of membrane sampled.
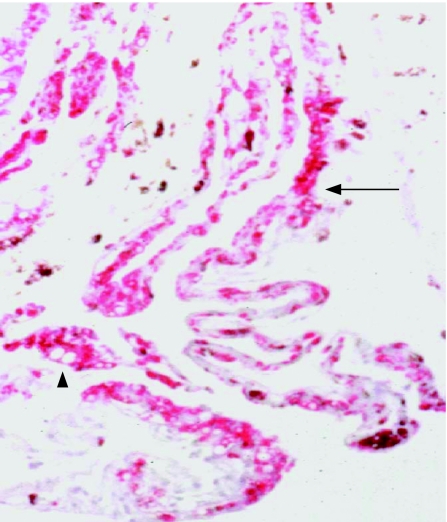
Figure 1 Immunochemistry of a retro‐oil epiretinal membrane (folded) showing a dense infiltration of macrophages (CD68 antibody (red), haematoxylin counterstain; arrow). Microglobules of silicone oil are present in macrophages (arrowhead). Original magnification ×200.
Table 3 Macrophage (CD68) and glial cell (glial fibrillary acidic protein ) staining of specimens.
| Specimen | Glial cell counts | Macrophage cell counts | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | Insufficient specimen | 0 | 1 | 2 | 3 | 4 | Insufficient specimen | |
| Controls, n = 37 | 7 | 6 | 6 | 5 | 6 | 7 | 36 | 1 | 0 | 0 | 0 | 0 |
| PVR ERMs, n = 14 | 3 | 4 | 2 | 1 | 1 | 3 | 5 | 4 | 3 | 1 | 1 | 0 |
| ERM under oil ,n = 33 | 7 | 1 | 9 | 7 | 4 | 5 | 2 | 4 | 2 | 6 | 19 | 0 |
| Retinectomy ,n = 19 | 0 | 2 | 3 | 2 | 9 | 3 | 13 | 5 | 0 | 0 | 0 | 1 |
| Retinectomy under oil, n = 14 | 0 | 1 | 2 | 2 | 6 | 3 | 4 | 3 | 3 | 1 | 3 | 0 |
ERM, epiretinal membrane; PVR, proliferative vitreoretinopathy.
Grade represents the average number of cells per five high‐power fields. 0, no cells present. Insufficient specimen refers to tissue samples too small to grade accurately.
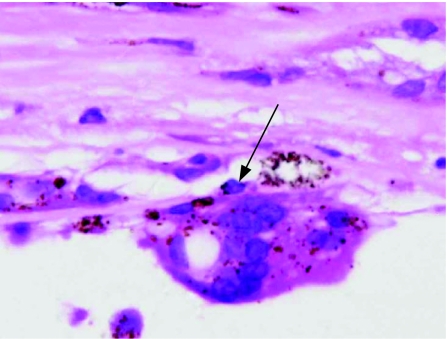
Figure 2 Light micrograph (haematoxyline and eosin) showing a giant cell in a retro‐oil retinectomy specimen (arrow). Original magnification ×400.
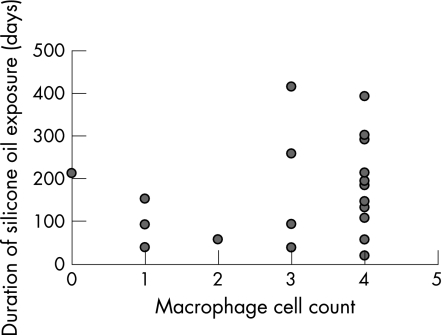
Clinical data were available for 24 cases. A scatter diagram plotting duration of exposure to silicone oil against grade of macrophage response showed no direct relationship between the two variables (fig 3); a grade 4 macrophage response was observed in a patient exposed to silicone oil for 18 days. An intense macrophage response (grade 4) was present in five of the six patients who had had silicone oil removed previously and had no oil in situ at the time the specimen was taken.
Figure 3 Scatter diagram showing the relationship between macrophage cell counts and duration of exposure to silicone oil in retro‐oil epiretinal membranes.
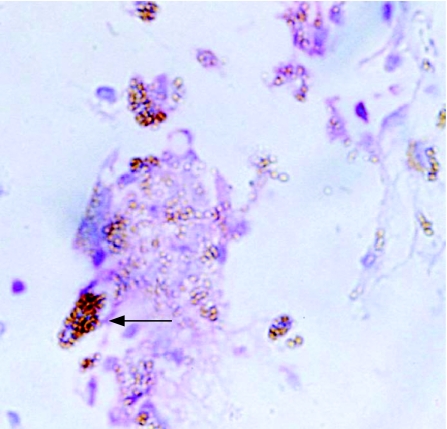
Moderate numbers of glial cells were present in these membranes (table 3), which was similar to that noted in the control ERMs (p = 0.442, χ2 test), but greater than that observed in the PVR membranes. Two specimens showed positive staining for cytokeratin markers. One membrane had a low‐grade T lymphocyte response, which was associated with an area of macrophage infiltrate (fig 4). No samples were positive for B lymphocytes.
Figure 4 Immunochemistry of a retro‐oil epiretinal membrane with a T lymphocyte infiltrate (CD45 antibody (red), haematoxylin and eosin counterstain; arrow). Original magnification ×400.
No silicone oil
ERMs from patients with PVR with no exposure to silicone oil showed little or no macrophage response. In 9 of 14 PVR membranes, the macrophage numbers were graded as 0 or 1 (table 3). One specimen had a low‐grade T lymphocyte infiltrate, which was closely associated with macrophages. Of the 14 PVR membranes, 1 was positive for RPE cell markers, none were positive for B lymphocytes. Small numbers of glial cells were seen in 6 of 11 specimens (3 had insufficient specimen available for analysis), with a more prominent glial content (grade 3 or 4) in 2. PVR membranes differed from control membranes in the degree of pigmentation—in PVR membranes, pigmentation was of greater density and more widespread distribution compared with that seen in control tissue.
Retinectomy specimens
With silicone oil
The morphology of retinal specimens was generally of good quality, with well‐preserved retinal architecture. In 5 of the 14 specimens, areas of disorganised retina were observed. ERMs were present on the retinal surface of 12 of the 14 retinectomyspecimens.
Macrophages were confined to ERMs in 10 of the 14 retrosilicone oil retinectomy specimens. In the other four specimens, macrophage distribution was more widespread, extending into disorganised, gliotic retina (fig 5). In two of these four specimens, silicone oil, most of which had been phagocytosed by macrophages, was also present in gliotic retina. The variation in macrophage cell counts generally reflected the extent of ERM formation on the retinal surface—that is, little or no macrophage response was seen if there was minimal ERM in the section sampled. Giant cells were found in ERMs, but were not observed in the retina. Staining was negative for B and T lymphocytes and cytokeratin markers. Glial cell up regulation was observed in all the samples with sufficient specimen present (n = 11).
Figure 5 Immunochemistry of a retinectomy specimen showing gliotic retina with macrophage infiltration (arrow). Original magnification ×200.
No silicone oil
When present, macrophages were observed in ERMs. Retinectomy specimens not exposed to silicone had a significantly lower macrophage count than the oil group (p<0.001, χ2 test). All 18 specimens available for analysis had a macrophage grade of between 0 and 1. Stains for T and B lymphocytes and RPE were negative in all specimens. Optically empty spaces similar to those found in the silicone oil group were noted in the retinal architecture of some retinectomy specimens. Optically, empty spaces were distinguishable from intraretinal silicone oil by the absence of eccentric granules of pigment. Glial fibrillary acidic protein staining of grade 3 or 4 was observed in 11 of 16 specimens with sufficient tissue for analysis.
Controls
Non‐retinal detachment ERM specimens were negative for macrophages, T and B lymphocytes. One specimen showed low‐grade staining for cytokeratin (RPE cells), and this patient had undergone previous vitreoretinal surgery for a macular hole before subsequent ERM removal. Positive staining on inflammatory controls verified the efficacy of each primary antibody. Twenty‐three membranes were noted to have a glial component; the grade of glial up regulation varied between 1 and 4.
Discussion
This study has shown that the intraocular inflammatory response associated with silicone oil differs from that seen in eyes with similar pathologies but no silicone oil. Macrophage cell counts were significantly greater in ERMs with a history of exposure to silicone oil (p<0.001). This intense inflammatory response seems to be mediated by bloodborne macrophages—negative staining for cytokeratin markers providing evidence that the macrophages were not transformed RPE cells. Macrophages often contained phagocytosed silicone oil and seemed to remain viable despite large volumes of silicone oil in the cell, a finding also noted in other studies.18,26 The multinucleated giant cells observed in this study have been documented in previous studies and suggest a chronic granulomatous inflammatory response to intraocular silicone oil.16,18
Previous work has shown that the infiltration of silicone oil in ocular tissues starts at an early stage after intraocular injection,26 and our study supports this finding, silicone oil being found in epiretinal membranes within 1 month of silicone oil exchange. Beyond this early time point, the intensity of the inflammatory response seemed unrelated to the duration of exposure. Marked inflammation was also present despite previous removal of silicone oil. This supports the view that although silicone oil may initiate an inflammatory response, this may continue despite oil removal and contribute to ongoing complications such as raised intraocular pressure.17 The small amount of emulsified oil that may remain after removal of the bulk of the oil could also potentially contribute to ongoing inflammation. The early appearance of silicone oil in ERMs, the lack of association between duration of tamponade and intensity of inflammation, and the persistence of inflammation after silicone oil removal suggest that the use of silicone oil in itself is of greater importance than the duration of intraocular tamponade.
The presence of inflammatory cells in PVR periretinal membranes in the absence of exposure to silicone oil has been described in a number of reports.19,20,21,23,25,27 Our study found small numbers of macrophages in 7 of the 14 membranes and a more intense macrophage response in 2. T lymphocytes were observed in only 1 of 14 ERMs we examined. We also noted T lymphocytes in only 1 of 33 ERMs exposed to silicone oil, suggesting that a cellular immune response to intraocular silicone oil is, if present, of a low intensity. In a study of 32 membranes by Nicolai and Eckardt,27 T lymphocytes were found in 19 of 23 retro‐oil epiretinal membranes, but were not observed in PVR membranes not exposed to silicone oil (n = 15). Other previous reports have documented low (often variable) numbers of T lymphocytes in PVR ERMs, although the exposure to silicone oil of the specimens in these studies is often not documented.17,19,20,21,23,25 Differing fixation regimens and immunohistochemical staining techniques may have affected the sensitivity of this and previous studies to detect the presence of T lymphocytes in PVR fibrocellular membranes. Additionally, the type of silicone oil used may influence the T lymphocyte response; older, less purified silicone oil may elicit a more marked immune response compared with newer, highly purified silicone oils. This may bias previous studies towards high T lymphocyte levels; however, even small numbers of T lymphocytes may potentially play a part in the proliferative response leading to PVR membrane formation.28 Overall, the findings of this study suggest that T lymphocytes are a minor and infrequent component of PVR membranes and are not markedly increased by the presence of silicone oil. The absence of B lymphocytes in both PVR membranes and silicone oil membranes suggests that humoral immune responses do not play a major part in the wound healing response resulting in PVR membrane formation, or in the response to the presence of silicone oil. Humoral immune system responses have been observed in PVR membranes in a previous study,25 but this observation has not, in general, been replicated in subsequent work.
It is notable that in this study, oil was much more prominent in ERMs than in the retina. This supports the findings of two previous studies which have also observed that tissue infiltrated with silicone oil is usually confined to the retinal surface.29,30 In the retinectomy oil specimens, intraretinal silicone oil was observed only when the retinal architecture was severely disorganised, suggesting that retinal injury is a prerequisite for oil infiltration. Kirchof et al,31 in a case series of eight eyes, reported that intraretinal silicone oil was absent unless accompanied by subretinal oil. In addition, animal studies did not find silicone oil in the outer retinal layer unless injected subretinally,32 and it has been postulated that this may be because of the integrity of the outer limiting membrane.32
Overall, these findings suggest that although oil accumulation in ERMs is common, it is relatively rare in the retina and it may require predisposing features (notably retinal injury) for retinal penetration. This also suggests that emulsified oil may be incorporated into ERMs during their formation (and oil may stimulate membrane formation).33 The lack of oil infiltration to intact retina, even after long‐term tamponade, supports the view that retinal toxicity with modern silicone oils is limited, or that if there is toxicity then this is indirect.
In conclusion, this study has shown that intraocular silicone oil produces an inflammatory reaction, primarily mediated by bloodborne macrophages. This response can be observed within 1 month after silicone oil injection and continues after silicone oil removal. Silicone oil infiltrates ERMs, but seems to have only limited retinal penetration confined to areas of retinal injury.
Abbreviations
ERM - epiretinal membrane
HPF - high power fields
PVR - proliferative vitreoretinopathy
RPE - retinal pigment epithelium
Footnotes
Funding: None.
Competing interests: None declared.
References
- 1.Cibis P A, Becker B, Okun E.et al The use of liquid silicone in retinal detachment surgery. Arch Ophthalmol 196268590–599. [DOI] [PubMed] [Google Scholar]
- 2.Leaver P. Complications of intraocular silicone oil. In: Ryan SJ, Glaser BM, eds. Surgical retina. 2nd edn. St Louis, MO: Mosby, 19942165–2179.
- 3.Watzke R C. Silicone retinopoesis for retinal detachment: a long‐term clinical evaluation. Arch Ophthalmol 196777185–196. [DOI] [PubMed] [Google Scholar]
- 4.Abrams G W, Azen S, McCuen B.et al Vitrectomy with silicone oil or long‐acting gas in eyes with severe proliferative vitreoretinopathy: results of additional long‐term follow up. Silicone Study Report 11. Arch Ophthalmol 1997115335–344. [DOI] [PubMed] [Google Scholar]
- 5.Foulks G, Hatchell D, Proia A.et al Histopathology of silicone oil keratopathy in humans. Cornea 19911029–37. [PubMed] [Google Scholar]
- 6.Honavar S, Goyal M, Majji A.et al Glaucoma after pars plana vitrectomy and silicone oil injection for complicated retinal detachments. Ophthalmology 1999106169–177. [DOI] [PubMed] [Google Scholar]
- 7.Budde M, Cursiefen C, Holbach L M.et al Silicone oil—associated optic nerve degeneration. Am J Ophthalmol 2001131392–394. [DOI] [PubMed] [Google Scholar]
- 8.Srinivasan A, Singh A K, Desai S P.et al Foreign body episcleral granulomas complicating intravitreal silicone oil tamponade—a clinicopathological study. Ophthlamology 20031101837–1840. [DOI] [PubMed] [Google Scholar]
- 9.Eckle D, Kampik A, Hintschich C.et al Visual field defect in association with chiasmal migration of intraocular silicone oil. Br J Ophthalmol 200589918–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ni C, Wang W, Albert D.et al Intravitreous silicone injection—histopathological findings in a human eye after 12 years. Arch Ophthalmol 19831011399–1401. [DOI] [PubMed] [Google Scholar]
- 11.Chung J, Spaide R. Intraretinal silicone oil vacuoles after macular hole surgery with internal limiting membrane peeling. Am J Ophthalmol 2003136766–767. [DOI] [PubMed] [Google Scholar]
- 12.Donahue S P, Friberg T R, Johnson B L. Intraconjunctival cavitary inclusions of silicone oil complicating retinal detachment repair. Am J Ophthalmol 1992114639–640. [DOI] [PubMed] [Google Scholar]
- 13.Eller A, Friberg T, Mah F. Migration of silicone oil into the brain: a complication of intraocular silicone oil for retinal tamponade. Am J Ophthalmol 2000129685–688. [DOI] [PubMed] [Google Scholar]
- 14.Shaikh S, Egbert P R, Goldblumet al Granulomatous local cell reaction to intravitreal silicone. Arch Ophthalmol 20001181133–1134. [PubMed] [Google Scholar]
- 15.Knorr H L J, Seltsam A, Holbach L M.et al Intraocular silicone oil: a clinicopathological study of 36 enucleated eyes. Ophthalmologe 199693130–138. [PubMed] [Google Scholar]
- 16.Parmley V C, Barishak Y R, Howes E L.et al Foreign‐body giant cell reaction to liquid silicone. Am J Ophthalmol 1986101680–683. [DOI] [PubMed] [Google Scholar]
- 17.Heidenkummer H P, Messmer E M, Kampik A. Recurrent vitreoretinal membranes during intravitreal silicone oil tamponade. Morphological and immunohistochemical investigations. Ophthalmologe 199693121–125. [PubMed] [Google Scholar]
- 18.Betis F, Leguay J M, Hofman P. Multinucleated giant cells in periretinal silicone granulomas are associated with progressive proliferative vitreoretinopathy. Eur J Ophthalmol 200313634–641. [DOI] [PubMed] [Google Scholar]
- 19.Jerden J, Pepose J, Michels R.et al Proliferative vitreoretinopathy membranes—an immunohistochemical study. Ophthalmology 198996801–810. [DOI] [PubMed] [Google Scholar]
- 20.Hiscott P, Unger W, Grierson I.et al The role of inflammation in the development of epiretinal membranes. Curr Eye Res 19887877–892. [DOI] [PubMed] [Google Scholar]
- 21.Hiscott P, Grierson I, McLeod D. Natural history of fibrocellular epiretinal membranes: a quantitative, autoradiographic and immunohistochemical study. Br J Ophthalmol 198569810–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charteris D G, Hiscott P, Robey H L.et al Inflammatory cells in proliferative vitreoretinopathy subretinal membranes. Ophthalmology 199310043–46. [DOI] [PubMed] [Google Scholar]
- 23.Charteris D G, Hiscott P, Grierson I.et al Proliferative vitreoretinopathy–lymphocytes in epretinal membranes. Ophthalmology 1992991364–1367. [DOI] [PubMed] [Google Scholar]
- 24.Heidenkummer H P, Kampik A. Intercellular adhesion molecule‐1 (ICAM‐1) and leukocyte function‐associated antigen‐1 (LFA‐1) expression in human epiretinal membranes. Graefes Arch Clin Exp Ophthalmol 1992230483–487. [DOI] [PubMed] [Google Scholar]
- 25.Baudouin C, Fredj‐Reygrobellet D, Gordon W.et al Immunohistologic study of epiretinal membranes in proliferative vitreoretinopathy. Am J Ophthalmol 1990110593–598. [DOI] [PubMed] [Google Scholar]
- 26.Bornfeld N, El‐Hifnawi E, Laqua H. Ultrastructural characteristics of preretinal membranes from human eyes filled with silicone oil. Am J Ophthalmol 1987103770–775. [DOI] [PubMed] [Google Scholar]
- 27.Nicolai U, Eckardt C. Immunohistochemical findings of epiretinal membranes after silicone oil injection. Fortschr Ophthalmol 199188660–664. [PubMed] [Google Scholar]
- 28.Charteris D G. Surgical management of HIV related retinal detachment. Br J Ophthalmol 199781177–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis H, Burke J M, Abrams G W.et al Perisilicone proliferation after vitrectomy for proliferative vitreoretinopathy. Ophthalmology 198895583–591. [DOI] [PubMed] [Google Scholar]
- 30.Eckardt C, Nicolai U, Czank M.et al Identification of silicone oil in the retina after intravitreal injection. Retina 199212S17–S22. [DOI] [PubMed] [Google Scholar]
- 31.Kirchhof B, Tavakolian H, Heimann K. Histopathological findings in eyes after silicone oil injection. Graefes Arch Clin Exp Ophthalmol 198622434–37. [DOI] [PubMed] [Google Scholar]
- 32.Shikishima K, Ohki K, Machi N.et al Effects and distribution of intravitreally or subretinally injected silicone oil identified in rabbit retina using osmium tetroxide method. Jpn J Ophthalmol 199236469–478. [PubMed] [Google Scholar]
- 33.Asaria R H, Kon C H, Bunce C.et al Silicone oil concentrates fibrogenic growth factors in the retro‐oil fluid. Br J Ophthalmol 2004881439–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]