Abstract
Aim
To evaluate the responsiveness of the Vision core module 1 (VCM1) vision‐related quality of life (VR‐QOL) questionnaire to changes in visual acuity in patients with posterior and intermediate uveitis and to validate its use as a clinical end point in uveitis.
Methods
Logarithm of the minimum angle of resolution visual acuity and VR‐QOL using the VCM1 questionnaire were prospectively recorded in 37 patients with active posterior segment intraocular inflammation before starting systemic immunosuppression with ciclosporin, tacrolimus or the anti‐tumour necrosis factor (TNF) agent, p55TNFr‐Ig, and again 3 months later. Spearman analysis was used to correlate improvements in visual acuity and VR‐QOL between baseline and 3 months.
Results
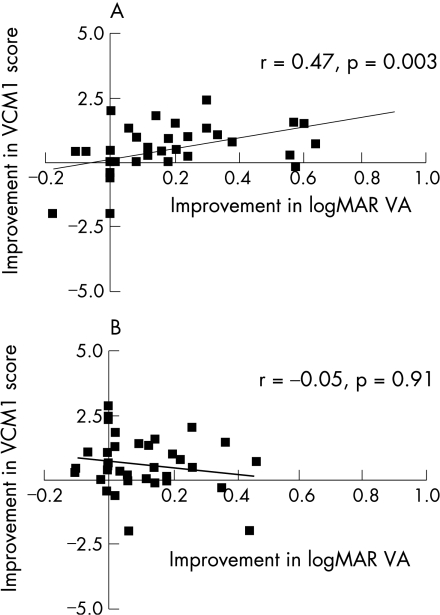
The correlation between changes in visual acuity and VR‐QOL was moderate to good for the worse eye (r = 0.47, p = 0.003), but poor for the better eye (r = −0.05, p = 0.91). The responsiveness indices effect size and standardised response mean were 0.57 and 0.59, respectively, showing that the VCM1 questionnaire is moderately responsive to immunsosuppressive therapy for active uveitis.
Conclusion
Changes in VR‐QOL measured with the VCM1 questionnaire correlated moderately well with changes in the worse eye visual acuity, suggesting that the VCM1 is a valid instrument for monitoring response to treatment in uveitis.
Vision‐related quality of life (VR‐QOL) questionnaires have been established as useful tools for measuring patients' perceptions of their vision in a variety of diseases, and are now used to evaluate treatment efficacy in clinical trials in ophthalmology. In the case of uveitis, accurately quantifying the response to treatment represents a major challenge, and the best method for measuring this in clinical trials is currently being reviewed by an international workshop for uveitis.1 By combining objective measures of inflammation, such as visual acuity and vitreous haze, with a subjective assessment of vision using VR‐QOL in a composite scoring system, it might be possible to improve the reliability and validity of grading changes in uveitis activity with treatment.1,2 Although VR‐QOL has been shown to correlate with visual acuity in uveitis,3,4,5 the responsiveness of VR‐QOL to changes in vision over time, and consequently its validity as a clinical end point, has not been evaluated in uveitis.
To explore the relationship between visual acuity and subjective visual function in uveitis, we prospectively measured visual acuity and VR‐QOL in patients undergoing treatment for active posterior segment intraocular inflammation over a 3‐month period. This study aimed to evaluate the validity (the extent to which an instrument measures what it is intended to measure) of using VR‐QOL to measure changes in vision in uveitis by correlating changes in VR‐QOL with changes in logarithm of the minimum angle of resolution (logMAR) visual acuity, the gold standard measure of vision and arguably the best available surrogate marker of intraocular inflammation.
Patients and methods
In all, 37 patients with non‐infectious posterior uveitis, intermediate uveitis or panuveitis participated in this study. All patients were enrolled in one of two clinical trials of immunosuppressive therapies for uveitis; a prospective randomised trial of ciclosporin versus tacrolimus treatment or a phase I/II study of a tumour necrosis factor (TNF) inhibitor, p55TNFr‐Ig. The details and results of these clinical trials have been described elsewhere.6,7,8 At the beginning of the study, all patients had active posterior segment intraocular inflammation that was refractory to oral prednisolone (ciclosporin v tacrolimus study) or oral prednisolone and at least one immunosuppressive agent (anti‐TNFα therapy study). A total of 26 (70.3%) patients were treated orally with either tacrolimus or ciclosporin and 11 (29.7%) patients received a single dose of p55TNFr‐Ig as an intravenous infusion. Approval for the study was provided by the hospital ethics board and informed consent obtained from all patients. The study was performed in two tertiary referral centres for inflammatory eye disease in the UK, Bristol Eye Hospital, Bristol, and Aberdeen Royal Infirmary, Aberdeen.
VR‐QOL and visual acuity were assessed immediately before enrolment in the clinical trials and after 3 months. All participants in the clinical trials described above with a record of VR‐QOL and visual acuity at baseline and 3 months later were included in this study. VR‐QOL was measured using the Vision core module 1 (VCM1) Questionnaire, a 10‐item questionnaire that provides a subjective assessment of concern regarding vision, with scores ranging from 0 (best score) to 5 (worst score) with 50 intervals. The VCM1 questionnaire was designed for use in a wide variety of eye diseases.9 It deals with how visual impairment evokes feelings of embarrassment, frustration, isolation and sadness, and measures patients' concern about their vision, its effect on their personal safety and coping in everyday life and how much their vision interferes with daily activities. The reliability of the VCM1 for measuring VR‐QOL is high (coefficient α = 0.93).9 The questionnaire was self‐administered in approximately 5 min. Best‐corrected logMAR visual acuity scored for individual letters was measured at 4 m using the Early Treatment of Diabetic Retinopathy Study chart mounted on the Lighthouse Chart Illumination Unit.
Spearman's analysis was used to correlate improvements in visual acuity and VR‐QOL between baseline and 3 months. VR‐QOL in treatment responders and non‐responders was compared using the Mann–Whitney U test. The responsiveness of the VCM1 questionnaire to treatment and the accompanying change in vision was evaluated by calculating the effect size and the standardised response mean (SRM) for the group.10 The effect size was defined as the mean change in the VCM1 score at 3 months divided by its standard deviation (SD) at baseline, and reflects the magnitude of the change in VCM1 in response to the treatment started at baseline. Cohen11 defines an effect size of 0.2 as a small change, 0.5 a medium change and ⩾0.8 a large change. The SRM was defined as the mean change in the VCM1 score between baseline and 3 months for the group divided by the standard deviation of the change in score over the same period.
Results
Table 1 shows the clinical and demographic characteristics of the patients. None of the patients had any coexisting ocular disease that was unrelated to uveitis. In general, they had moderate to severe uveitis; this was reflected by the need for second‐line immunosuppression to control intraocular inflammation in all patients. Table 2 describes the visual acuity and VR‐QOL scores at baseline and 3 months later.
Table 1 Clinical and demographic characteristics of the patients.
| Age (years) | |
| Mean | 45 |
| Range | 19–67 |
| Sex | |
| Male | 13 |
| Female | 24 |
| Uveitis laterality | |
| Bilateral | 29 |
| Unilateral | 8 |
| Uveitis duration (years) | |
| Mean | 5 |
| Range | 0.1–15 |
| Anatomical uveitis diagnosis | |
| Intermediate | 12 |
| Posterior | 4 |
| Panuveitis | 21 |
| Uveitis aetiology | |
| Idiopathic | 28 |
| Sarcoidosis | 2 |
| Behçet's disease | 2 |
| Sympathetic ophthalmia | 1 |
| TINU syndrome | 1 |
| Presumed ocular histoplasmosis | 1 |
| Punctate inner choroidopathy | 1 |
| Serpiginous choroiditis | 1 |
| Treatment added at baseline | |
| Ciclosporin | 10 |
| Tacrolimus | 16 |
| Anti‐TNFα therapy* | 11 |
TINU, tubulointerstitial nephritis and uveitis; TNF, tumour necrosis factor.
*TNFα receptor fusion protein, p55TNFr‐Ig.
Table 2 Log MAR visual acuity and vision‐related quality of life before enrolment in study and 3 months after systemic immunosuppression.
| Baseline n (%) | 3 months n (%) | |
|---|---|---|
| VA—worse eye | ||
| 0–0.28 | 12 (32) | 20 (54) |
| 0.3–0.58 | 7 (19) | 5 (14) |
| 0.6–0.78 | 5 (14) | 1 (3) |
| 0.8–1 | 2 (5) | 5 (14) |
| >1 | 11 (30) | 6 (16) |
| VA—better eye | ||
| 0–0.28 | 25 (68) | 29 (78) |
| 0.3–0.58 | 7 (19) | 6 (16) |
| 0.6–0.78 | 2 (5) | 1 (3) |
| 0.8–1 | 3 (8) | 1 (3) |
| >1 | 0 (0) | 0 (0) |
| VCM1 score | ||
| 0–1 | 9 (24) | 20 (54) |
| 1.1–2 | 13 (35) | 7 (19) |
| 2.1–3 | 11 (30) | 9 (24) |
| 3.1–4 | 4 (11) | 1 (3) |
| 4.1–5 | 0 (0) | 0 (0) |
VA, visual acuity; VCM1, vision core module 1.
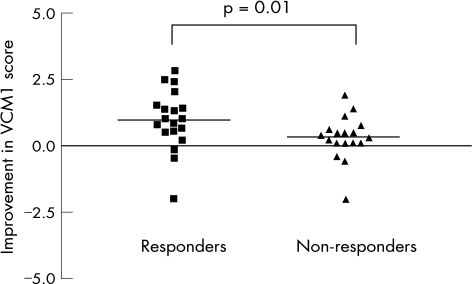
Visual acuity improved in at least one eye by ⩾2 lines between baseline and at the 3‐month follow‐up visit in 19 (51.4%) patients, and did not decrease by 2 lines or more in any of the patients. The VCM1 Score improved by at least 1.0 in 13 (35.1%) patients over the same period. Figure 1 shows the relationship between the improvements in the VCM1 Score and visual acuity for the worse eye and better eye between baseline and 3 months. The correlation between changes in visual acuity and VR‐QOL was moderate to good for the worse eye (r = 0.47, p = 0.003), but poor for the better eye (r = −0.05, p = 0.91). Using an arbitrarily chosen definition of treatment response—an improvement in visual acuity in either eye of at least 2 lines between baseline and 3 months—we found a significantly greater improvement in VCM1 score in treatment responders compared with non‐responders, as shown in fig 2 (median improvement = 1 for responders and 0.25 for non‐responders; p = 0.01). The effect size and SRM, indices of the responsiveness of the VCM1 to the change in treatment initiated at baseline, were 0.57 and 0.59, respectively, indicating that the VCM1 questionnaire shows moderate to high responsiveness to immunsosuppressive therapy for uveitis.
Figure 1 Correlation of improvements in logarithm of the minimum angle of resolution (logMAR) visual acuity (VA) and vision‐related quality of life vision core module 1 (VCM1) between baseline and 3 months for the worse eye (A) and the better eye (B).
Figure 2 A comparison of the improvement in vision core module 1 (VCM1) Score between baseline and 3 months in treatment responders and non‐responders (median = 1 and 0.25, respectively; p = 0.01). Treatment response was defined as an improvement in visual acuity of at least 2 lines (0.2 logarithm of the minimum angle of resolution) over the same period. The horizontal lines represent the median for each dataset.
Discussion
The measurement of patient‐centred outcomes such as VR‐QOL supports the holistic approach to caring for patients with eye disease and helps us to understand more about the effect of visual impairment on patients' physical, mental and social functioning. Quality‐of‐life instruments can also aid clinical decision making which otherwise focuses mainly on clinical parameters such as visual acuity. This study shows that changes in patients' perceptions of their vision over time correlate with changes in visual acuity in posterior segment intraocular inflammatory disease. In addition, the responsiveness indices effect size and SRM show that the VCM1 questionnaire is moderately responsive to immunosuppressive therapy for active uveitis. The recently published guidelines on standardisation of uveitis nomenclature provide a unified classification of uveitis and some definitions of success to aid reporting studies on uveitis.1 However, despite this, clinical trials of uveitis remain hampered by semiquantitative assessments of activity that are open to considerable observer bias. Our findings confirm the usefulness of VR‐QOL as an outcome measure in clinical trials of uveitis treatment and indicate that the development of a quantitative scoring system for uveitis which incorporates VR‐QOL would be of benefit. In addition, the ease of administration and interpretation of the VCM1 supports its use in routine practice where it can complement clinical assessment and aid patient management.
The correlation between VCM1 and the worse eye visual acuity, but not the better eye visual acuity, is consistent with our findings in a study of visual function and quality of life in intermediate uveitis.3 By contrast, VR‐QOL analyses in patients with cataract, corneal transplantation and age‐related macular degeneration have found a stronger correlation with the better eye visual acuity.12,13,14 A possible explanation for this difference is the relapsing‐remitting nature of posterior uveitis and the heightened awareness patients have for fluctuations in their worse eye vision. Visual impairment in cataract and macular degeneration is chronic and usually steadily progressive, possibly leading to greater concern for maintaining vision in the better eye.
Although visual acuity is probably the most reliable single measure of posterior uveitis activity, in many patients, it fails to improve despite effective control of intraocular inflammation because of irreversible damage to the macula or optic nerve. Visual acuity is only one aspect of vision and improvements in visual field or contrast sensitivity can lead to subjective improvements in vision without any change in visual acuity; hence the benefit of using more global outcome measures. This may explain why the correlation between VR‐QOL and visual acuity was not higher.
In summary, changes in VR‐QOL measured with the VCM1 questionnaire correlate with changes in visual acuity in uveitis, suggesting that the VCM1 is a valid instrument for monitoring response to treatment in this disease. This finding supports the use of VR‐QOL of life as a treatment end point both in routine clinical practice and in clinical trials of uveitis treatments.
Abbreviations
log MAR - logarithm of the minimum angle of resolution
SRM - standardised response mean
TNF - tumour necrosis factor
VCM1 - vancomycin 1
VR‐QOL - vision‐related quality of life
Footnotes
Funding: This study was supported by Fujisawa and the National Eye Research Centre, UK.
Competing interests: None.
References
- 1.Jabs D A, Nussenblatt R B, Rosenbaum J T.et al Standardization of uveitis nomenclature for reporting clinical data. Results of the first international workshop. Am J Ophthalmol 2005140509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenbaum J T, Deodhar A, Suhler E B.et al How do you know? Br J Ophthalmol 200488980–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy C C, Hughes E H, Frost N A.et al Visual function and quality of life in intermediate uveitis. Br J Ophthalmol 2005891161–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gardiner A M, Armstrong R A, Dunne M C.et al Correlation between visual function and visual ability in patients with uveitis. Br J Ophthalmol 200286993–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schiffman R M, Jacobsen G, Whitcup S M. Visual function and general health status in patients with uveitis. Arch Ophthalmol 2001119841–849. [DOI] [PubMed] [Google Scholar]
- 6.Murphy C C, Greiner J, Plskova J.et al Prospective randomized trial of cyclosporin versus tacrolimus therapy for posterior and intermediate uveitis. Arch Ophthalmol 2005123634–641. [DOI] [PubMed] [Google Scholar]
- 7.Murphy C C, Greiner K, Plskova J.et al Neutralizing TNF activity leads to remission in refractory non‐infectious uveitis. Arch Ophthalmol 2004122845–851. [DOI] [PubMed] [Google Scholar]
- 8.Greiner K, Murphy C C, Plskova J.et al Anti‐TNFα therapy modulates the phenotype of peripheral blood CD4+ T cells in patients with posterior segment intraocular inflammation. Invest Ophthalmol Vis Sci 200445170–176. [DOI] [PubMed] [Google Scholar]
- 9.Frost N A, Sparrow J M, Durant J S.et al Development of a questionnaire for measurement of vision‐related quality of life. Ophthalmic Epidemiol 19985185–210. [DOI] [PubMed] [Google Scholar]
- 10.Guyatt G, Walter S, Norman G. Measuring change over time: assessing the usefulness of evaluative instruments. J Chronic Dis 198740171–178. [DOI] [PubMed] [Google Scholar]
- 11.Cohen J.Statistical power analysis for the behavioural sciences, 2nd edn. Hillsdale, NJ: Lawrence Erlbaum Associates 1988
- 12.Steinberg E P, Tielsch J M, Schein O D.et al The VF‐14: an index of functional impairment in patients with cataract. Arch Ophthalmol 1994112630–638. [DOI] [PubMed] [Google Scholar]
- 13.Musch D C, Farjo A A, Meyer R F.et al Assessment of health‐related quality of life after corneal transplantation. Am J Ophthalmol 19971241–8. [DOI] [PubMed] [Google Scholar]
- 14.Miskala P H, Bressler N M, Meinert C L. Relative contributions of reduced vision and general health to NEI‐VFQ scores in patients with neovascular age‐related macular degeneration. Arch Ophthalmol 2004122758–766. [DOI] [PubMed] [Google Scholar]