Abstract
Aim
To evaluate secondary glaucoma after penetrating keratoplasty with anterior‐segment optical coherence tomography (OCT).
Design
Case series.
Methods
Four eyes of four patients with corneal opacity and increased intraocular pressure (IOP) were evaluated using high‐speed (2000 axial scans/s) OCT at 1.3 μm wavelength. Cross‐sectional images of the anterior segment were analysed to assess the cause of increase in pressure.
Results
Slit‐lamp evaluation of the anterior chamber in all cases was limited by corneal opacity. The OCT imaging allowed visualisation of anterior‐segment structures behind the opaque corneas. Using OCT, iris–intraocular lens adhesion and pupillary block were identified as the probable reasons for the increased IOP in one case. Peripheral anterior synechiae and angle closure were identified in the three remaining cases. In two cases, we found that the tip of the aqueous drainage tube was blocked by peripheral anterior synechiae.
Conclusions
OCT is similar to ultrasound in that it allows visualisation through opaque corneas. However, OCT has an advantage in that it requires neither contact nor immersion. It is a valuable tool for evaluating the depth of the anterior chamber angle and the causes of secondary angle closure.
Corneal diseases that require corneal transplantation are often associated with coexisting glaucoma; in fact, the presence of one disease entity can often predispose a patient to developing another. Patients who have undergone corneal transplantation have a greater risk of developing secondary glaucoma,1 and patients with pre‐existing glaucoma who have undergone corneal transplantation have a greater risk of developing glaucoma that remains refractory to medical management.2
Post‐keratoplasty glaucoma is a frequent cause of irreversible corneal decompensation and subsequent graft failure.1,3 There is often a delay in the diagnosis and management of these secondary types of glaucoma, as evaluating the type of glaucoma and measuring intraocular pressure (IOP) after penetrating keratoplasty can be challenging. Gonioscopic visualisation of the anterior chamber angle is often obstructed by corneal scars, oedema and opacities at the host–recipient interface.
Ultrasound imaging is often used to visualise intraocular structures when slit‐lamp biomicroscopy is blocked by opacities. In particular, ultrasound biomicroscopy (UBM) has been used to identify the anatomical characteristics of the anterior chamber angle.4 However, UBM has certain limitations. UBM studies need to be performed by highly trained individuals. An immersion bath is needed to couple the ultrasound from the transducer to the eye, which is time consuming and inconvenient to perform in the routine clinical setting. Use of the water bath also introduces an element of discomfort for the patient and poses a potential risk to the corneal graft.
Optical coherence tomography (OCT)5 is a new imaging modality that allows high‐resolution cross‐sectional imaging of the eye.6,7,8,9 Unlike ultrasound imaging, OCT requires no contact or immersion, the person acquiring the images requires minimal training and expertise, and it produces images with a higher spatial resolution. In this case series, we describe the use of OCT to visualise behind opaque corneas and assess the reasons for secondary angle closure. All cases involved increase in IOP after penetrating keratoplasty.
Methods
The study was carried out with the approval of the institutional review board of the University of Southern California, Los Angeles, California, USA. Informed consent was obtained from all participants, and the study was conducted in accordance with Health Insurance Portability and Accountability Act regulations.
We used an anterior‐segment OCT prototype provided by Carl Zeiss Meditec (Dublin, California, USA). The prototype is similar in performance to the Zeiss Visante model that has been recently approved by the US Food and Drug Administration. The OCT performance of the two models is virtually identical. The Visante is a more compact model, with slightly different features such as automated control of the chin rest. The prototype model uses a 1.3 μm wavelength light source to acquire 2000 axial scans/s. Each image frame includes 256 axial scans/image, giving an image acquisition rate of 8 frames/s. The scan geometry is telecentric (the OCT beam remains parallel to the fixation axis as it is scanned from side to side). The scan width is adjustable up to 16 mm and scan depths are either 4 or 8 mm in tissue. Axial resolution is measured to be 17 μm full‐width half‐maximum, assuming a corneal group index of 1.389.10 Images of the anterior segment are obtained with the subject in the sitting position. The head is stabilised by a chin/forehead rest, and the gaze is stabilised by an internal fixation target. The scanning module makes no physical contact with the patient's eye.
We identified three cases (three eyes of three patients) of marked increase in IOP after penetrating keratoplasty, in which corneal opacity prevented visualisation of the anterior segment. Informed consent was obtained from all patients, and OCT scanning was performed according to the research protocol approved by the University of Southern California Institutional Review Board.
Case reports
Case 1
A 62‐year‐old Asian man with a longstanding history of glaucoma presented for evaluation of acute increase in IOP in the right eye. He had undergone two Baerveldt glaucoma implants (Advanced Medical Optics, Santa Ana, California, USA) in each eye, and was receiving maximal medical treatment. The right eye had also undergone a corneal transplant for pseudophakic bullous keratopathy; however, the transplant had subsequently failed. Visual acuity was count fingers. The IOP was 32 mm Hg by Tonopen XL (Medtronic Solan, Jacksonville, Florida, USA). The firmness of the eye was confirmed by palpation. Corneal irregularity prevented the use of Goldmann applanation tonometry.
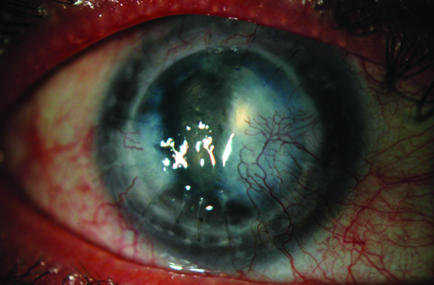
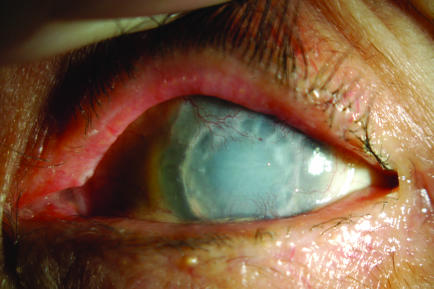
Slit‐lamp examination showed a thick, hazy graft, with areas of pannus and scarring (fig 1). The anterior chamber was shallow. The position of the tubes could not be visualised owing to corneal opacity and scarring at the graft–host interface. No bleb could be seen on clinical examination or on B‐scan ultrasonography.
Figure 1 Slit‐lamp photograph of patient 1 (right eye).
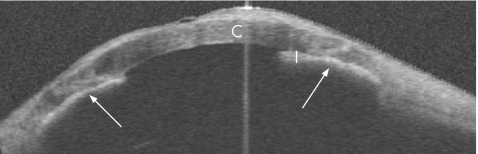
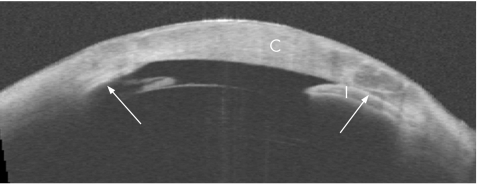
The OCT showed broad, confluent areas of Peripheral anterior synechiae (PAS: fig 2). Vertical scans showed thick sheets of PAS surrounding both drainage tubes along their entire length and down to the tips (fig 3). Although the lumen was patent, the opening was obstructed by PAS, blocking aqueous access to the tube.
Figure 2 Horizontal wide‐field (16×8 mm) optical coherence tomographic cross‐sectional image. Arrows point to peripheral anterior synechiae. C, cornea; I, Iris.
Figure 3 Three consecutive raster optical coherence tomographic images (7×4 mm each, 1 mm apart) of the anterior chamber showing the drainage tube in the superotemporal quadrant (arrows). The tube is surrounded by thick iris adhesions. Although the lumen is patent, the tip is obstructed by the peripheral anterior synechiae. C, Cornea; I, Iris.
The patient subsequently underwent repeat penetrating keratoplasty, with synechialysis, reformation of the anterior chamber and release of scar tissue around the tubes. The IOP normalised after surgery.
Case 2
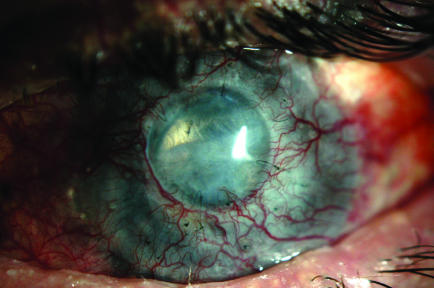
A 48‐year‐old Hispanic man with a longstanding history of Stevens–Johnson syndrome presented with reduced vision in the left eye. Visual acuity was 20/400. The right eye was phthisical after numerous surgeries. He had undergone multiple penetrating keratoplasties in the left eye, as well as placement of a Baerveldt glaucoma implant. Slit‐lamp examination showed moderate graft oedema, and an opaque and vascularised host corneal rim (fig 4). The anterior chamber was poorly visualised. The IOP was read as 81 mm Hg by Tonopen, but it was thought to be in the 30s by digital palpation. A review of the patient's history revealed a slow, progressive rise in IOP.
Figure 4 Slit‐lamp photograph of patient 2 (left eye).
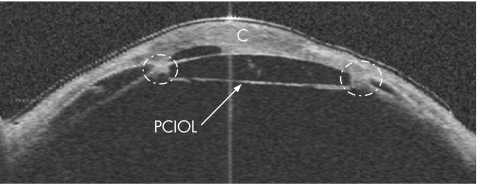
OCT showed extensive PAS and adhesion of the intraocular lens (IOL) to the iris in all four meridians, possibly causing an increase in IOP by pupillary block mechanism (fig 5). The IOL–iris diaphragm was pushed forwards against the cornea. The IOL was in contact with the cornea, probably causing an artefactual increase in the measured IOP and accounting for the extremely high Tonopen measurement.
Figure 5 Wide‐field (16×8 mm) optical coherence tomography cross‐sectional image at the 45° meridian. Dotted circles highlight adhesions between the posterior chamber intraocular lens (PCIOL), cornea (C) and iris.
The patient subsequently underwent IOL explantation, limbus‐to‐limbus penetrating keratoplasty and revision of the tube. The IOP is currently under good control.
CASE 3
An 88‐year‐old Asian patient with a history of advanced glaucoma presented to our clinic for evaluation of worsening vision and a slow, progressive rise in IOP in the left eye. Both eyes were pseudophakic and had undergone penetrating keratoplasty. Visual acuity was light perception in the left eye and the IOP was measured at 32 mm Hg by Tonopen. The firmness of the eye was confirmed by palpation. Slit‐lamp examination of the left eye showed a completely opaque corneal graft and rim with vascularisation (fig 6). There was no view of the anterior chamber. OCT showed an opaque, thickened cornea and extensive PAS in all four meridians (fig 7).
Figure 6 Slit‐lamp photograph of patient 3 (left eye).
Figure 7 Horizontal wide‐field (16×8 mm) optical coherence tomographic cross‐sectional image. Arrows point to peripheral anterior synechiae. C, cornea; I, Iris.
The patient is currently being managed medically as she is believed to have a poor prognosis for visual rehabilitation with further surgical intervention.
Discussion
Ophthalmic OCT was initially developed for retinal imaging,11,12 using a near‐infrared 0.8 μm wavelength. For anterior‐segment imaging, a longer wavelength of 1.3 μm confers two advantages: firstly, as 1.3 μm light is absorbed by water, retinal exposure is much lower, allowing the use of 20‐fold higher power without exceeding the retinal exposure limit.13 The higher power, in turn, permits much higher scan rates without sacrificing image quality.6,7,8,9 Secondly, the longer‐wavelength light scatters less in opaque tissues, allowing for deeper penetration. This permits imaging through the limbus to visualise angle structures such as the scleral spur and angle recess.6,7,8,9,14,15
This study tested the ability of high‐speed 1.3 μm OCT to obtain images through opaque corneas. We found that the OCT was able to image the anterior chamber and iris (including the posterior iris surface), even through severe corneal opacity (case 3). In all four cases, using the OCT images, we were able to deduce the reason for the acute increase in IOP, including anterior synechiae, posterior synechiae (with IOL) and obstruction of an anterior chamber drainage tube. Using OCT, we were able to survey the entire anterior segment from angle to angle in the wide‐angle mode along multiple meridians. A “raster” scan pattern, consisting of several parallel scans a short distance apart, was used to look at obstruction of the drainage tube (case 1).
OCT can deliver information about anatomical changes in the angle but not about tissue morphology or the specific aetiology for the anatomical changes. Although the OCT images did not provide definitive proof of the exact mechanism for increase in IOP, they did allow identification of altered anatomy that could, in turn, have been responsible for the increased IOP. Visualisation of these structures through opaque corneas would otherwise not have been possible. In each case, the OCT images helped guide our clinical and surgical decision making. The improved IOP control after surgical intervention, we believe, further supported our presumed diagnoses.
OCT cannot definitively distinguish between angle closure by PAS formation and appositional closure. However, given the clinical histories and slit‐lamp examination findings, along with the configuration of the iris–corneal touch on the images, we were more likely to suspect PAS formation in the pathophysiology of increased IOP in the above‐mentioned cases. The reduced IOP response after surgical intervention (eg, synechialysis in case 1) we believe further supports this.
Ultrasound imaging could also have been used to diagnose the underlying causes of glaucoma seen in our case series. In addition, ultrasound can penetrate beyond the iris to visualise rare causes of secondary glaucoma in the posterior chamber, such as ciliary body tumours. The post‐iris structures cannot be clearly visualised with OCT, but OCT is easier to use and is more comfortable for the patient, because it requires no fluid immersion. With recent Food and Drug Administration approval of the system (Carl Zeiss Meditec), anterior‐segment OCT may become more widely available for use in a variety of applications6,7,8,16 apart from evaluation of angle‐closure glaucoma.
To the best of our knowledge, these are the first reported cases of the use of OCT to evaluate secondary glaucoma in the setting of corneal opacity.
Abbreviations
IOL - intraocular lens
IOP - intraocular pressure
OCT - optical coherence tomography
PAS - peripheral anterior synechiae
UBM - ultrasound biomicroscopy
Footnotes
NIH R24 EY 13015 grant from National Eye Institute, Bethesda, Maryland, USA; research equipment and grant from Carl Zeiss Meditec, Dublin, California, USA; an unrestricted grant from Research to Prevent Blindness, New York, New York, USA.
Competing interests: DH receives royalty from OCT patents licensed to Carl Zeiss Meditec, Dublin, California, USA. D H and Y L receive research grant support from Carl Zeiss Meditec.
References
- 1.Irvine A R, Kaufman H E. Intraocular pressure following penetrating keratoplasty. Am J Ophthalmol 196968835–844. [DOI] [PubMed] [Google Scholar]
- 2.Lee R K, Fantes F. Surgical management of patients with combined glaucoma and corneal transplant surgery. Curr Opin Ophthalmol 20031495–99. [DOI] [PubMed] [Google Scholar]
- 3.Krontz D P, Wood T O. Corneal decompensation following acute angle‐closure glaucoma. Ophthalmic Surg 198819334–338. [PubMed] [Google Scholar]
- 4.Marchini G, Pagliarusco A, Toscano A.et al Biomicroscopic and conventional ultrasonographic study of ocular dimensions in primary angle‐closure glaucoma. Ophthalmology 19981052091–2098. [DOI] [PubMed] [Google Scholar]
- 5.Huang D, Swanson E A, Lin C P.et al Optical coherence tomography. Science 19912541178–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Radhakrishnan S, Rollins A M, Roth J E.et al Real‐time optical coherence tomography of the anterior segment at 1310 nm. Arch Ophthalmol 20011191179–1185. [DOI] [PubMed] [Google Scholar]
- 7.Radhakrishnan S, Huang D, Smith S D. Optical coherence tomography imaging of the anterior chamber angle. Ophthalmol Clin North Am 200518375–381. [DOI] [PubMed] [Google Scholar]
- 8.Radhakrishnan S, Goldsmith J, Huang D.et al Comparison of optical coherence tomography and ultrasound biomicroscopy for detection of narrow anterior chamber angles. Arch Ophthalmol 20051231053–1059. [DOI] [PubMed] [Google Scholar]
- 9.Hoerauf H, Winkler J, Scholz C.et al Transscleral optical coherence tomography—an experimental study in ex‐vivo human eye. Lasers Surg Med 200230209–215. [DOI] [PubMed] [Google Scholar]
- 10.Lin R, Shure M, Rollins A.et al Group index of the human cornea at 1.3 micron wavelength obtained in vitro by optical coherence domain reflectometry. Opt Lett 20042983–85. [DOI] [PubMed] [Google Scholar]
- 11.Swanson E A, Izatt J A, Hee M R.et al In vivo retinal imaging by optical coherence tomography. Opt Lett 1993181864–1866. [DOI] [PubMed] [Google Scholar]
- 12.Hee M R, Izatt J A, Swanson E A.et al Optical coherence tomography of the human retina. Arch Ophthalmol 1995113325–332. [DOI] [PubMed] [Google Scholar]
- 13.Laser Institute of America American National Standard for safe use of lasers. Orlando: Laser Institute of America, American National Standards Institute, 2000 [ANSI Z136, 1–2000].
- 14.Hoerauf H, Gordes R S, Scholz C.et al First experimental and clinical results with transscleral optical coherence tomography. Opthalmic Surg Lasers 200031218–222. [PubMed] [Google Scholar]
- 15.Wirbelauer C, Karandish A, Haberle H.et al Noncontact goniometry with optical coherence tomography. Arch Ophthalmol 2005123171–185. [DOI] [PubMed] [Google Scholar]
- 16.Huang D, Li Y, Rahakrishnan S.et al Corneal and anterior segment optical coherence tomography. In: Schuman JS, Puliafito CA, Fujimoto JG,eds. Optical coherence tomography of ocular diseases. 2nd edn. Thorofare, NJ: SLACK, 2004663–673.