Abstract
Objective
To evaluate the safety and efficacy of intravitreal bevacizumab (Avastin) as treatment for subfoveal choroidal neovascularisation (CNV) due to pathological myopia.
Methods
Consecutive series of primary or recurrent subfoveal CNV secondary to myopia treated with intravitreal bevacizumab 1.25 mg between August 2005 and January 2006 at the New England Eye Center, Boston, Massachusetts, USA, were reviewed retrospectively. Data from clinical examination, fundus photography, fluorescein angiography, optical coherence tomography and visual acuity were collected.
Results
There were 11 eyes of 9 patients. 5 of 11 eyes had been treated previously with photodynamic therapy. Pre‐injection visual acuity measured 20/50 to 20/100 in 6 eyes and 20/200 or worse in 5 eyes. After a mean follow‐up of 153 (range 35–224) days, post‐injection visual acuity measured 20/20 to 20/40 in 7 eyes, 20/50 to 20/100 in 1 eye and 20/200 or worse in 3 eyes. Three eyes received two bevacizumab injections and eight eyes received one injection. Visual acuity improved by a mean of +3.5 (range −1 to +8 lines) lines, and 8 of 11 eyes achieved 20/50 or better at the last follow‐up. Central foveal thickness improved from 340 (range 253–664) μm to 234 (range 142–308) μm, representing an average reduction of 103 (range +4 to −356) μm. No injection complications or drug‐related side effects were observed.
Conclusions
In this small series of eyes with limited follow‐up, intravitreal bevacizumab seems to be safe and potentially efficacious in eyes with subfoveal CNV secondary to pathological myopia.
Subfoveal choroidal neovascularisation (CNV) is a devastating complication of pathological myopia leading to immediate and potentially irreversible vision loss.1 Nearly 10% of eyes with degenerative retinal findings consistent with high myopia develop CNV.2 Photodynamic therapy (PDT) with verteporfin (Novartis, Duluth, Georgia, USA) is currently the only approved treatment for subfoveal CNV that has shown stabilisation of vision compared with placebo. At 1 year, 72% of treated eyes compared with 44% of placebo eyes lost <8 letters.3 However, no statistically significant treatment benefit was appreciated at 2 years.4
The introduction of pharmacological treatment that blocks vascular endothelial growth factor (VEGF) has resulted in new treatments of subfoveal CNV. An initial case report by Rosenfeld et al5 suggested that intravitreal bevacizumab (Avastin, Genentech, San Francisco, California, USA) 1 mg was safe and effective at treating subfoveal CNV secondary to age‐related macular degeneration (AMD) at 1 month. Avery et al6 published data on additional patients with subfoveal CNV secondary to AMD treated with intravitreal bevacizumab (1.25 mg). In 51 eyes with 8 weeks of follow‐up, median visual acuity improved from 20/200 to 20/80 without ocular side effects. However, limited information exists on the treatment of CNV secondary to pathological myopia with VEGF‐blocking agents. Nguyen et al7 reported on the first two patients in the literature with subfoveal CNV secondary to pathological myopia treated with intravenous bevacizumab (5 mg/kg). Visual acuity improved in two eyes, with retinal thickness and leakage on fluorescein angiography decreasing in all three eyes treated. This study was carried out to evaluate the effect of intravitreal bevacizumab for treating subfoveal CNV secondary to pathological myopia.
Methods
The charts of all patients who had undergone intravitreal bevacizumab treatment at the New England Eye Center, Boston, Massachusetts, USA, for the 6 months between August 2005 and January 2006 were reviewed. The institutional review board of Tufts‐New England Medical Center approved this study. Informed consent for the injection of bevacizumab was obtained from all patients. Only eyes treated for subfoveal CNV secondary to pathological myopia with a minimum of 1 month of follow‐up were included. Pathological myopia was defined as an eye with a minimum refractive error of −6 diopters or retinal signs of pathological myopia. Eyes with idiopathic CNV, AMD or angioid streaks were excluded. Evaluation with best‐corrected visual acuity, fundus photography, digital fluorescein angiography and macular scan with Stratus optical coherence tomography (OCT) V.4.0 (Carl Zeiss Meditec, Dublin, California, USA) were performed before the initial injection. Treatment was initiated only if there was evidence of an active CNV on the basis of the presence of leakage on fluorescein angiography and intraretinal or subretinal fluid on OCT. Bevacizumab 1.25 mg in a volume of 0.05 ml was formulated under sterile conditions and placed in a 1 ml syringe by the pharmacy of Tufts‐New England Medical Center. Before intraocular injection, tetracaine 0.5% was applied topically both as a drop and soaked in a pledget over the injection site, betadine 5% was irrigated on the conjunctival and corneal surface, and a lid speculum was placed. Using a 30‐gauge needle, 0.05 ml bevacizumab was injected into the vitreous cavity. Intraocular pressure was measured 15 min after the injection to ensure that the final eye pressure was ⩽30 mm Hg. Lowering of the intraocular pressure with anterior chamber paracentesis was not performed in any eyes. A topical antibiotic was used four times daily for 4 days. Follow‐up examinations occurred at routine intervals at the discretion of the treating ophthalmologist. Retreatment was based only on the presence of active leakage on fluorescein angiography or fluid collections imaged on OCT.
Results
Eleven eyes of nine patients (eight women and one man) received intravitreal bevacizumab 1.25 mg for active, subfoveal CNV secondary to pathological myopia (table 1).
Table 1 Characteristics at baseline and last follow‐up.
| Demographics | Preinjection status | Last follow‐up status | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient ID | Age (years) | Eye | Previous treatment | VA | RE (diopter) | CFT (μm) | Days | INJ | VA | CFT (μm) | Diff CFT (μm) |
| 1 | 70 | R | None | 20/70 | −5.50 | 253 | 35 | 1 | 20/50 | 142 | −111 |
| 2 | 57 | R | None | 20/60 | −7.25 | NA | 98 | 1 | 20/30 | 205 | NA |
| 3 | 65 | L | None | 20/400 | −9.00 | 664 | 110 | 1 | 20/200 | 308 | −356 |
| 4 | 38 | R | None | 20/400 | NA | 289 | 121 | 1 | 20/200 | 195 | −94 |
| 5 | 58 | L | PDTx2 | 20/200 | −10.00 | 269 | 147 | 2 | 20/400 | 273 | 4 |
| 6 | 44 | R | PDTx1 | 20/60 | −11.25 | 380 | 161 | 1 | 20/40 | 240 | −140 |
| 6 | 44 | L | PDTx1 | 20/70 | −12.50 | 373 | 175 | 1 | 20/40 | 270 | −103 |
| 7 | 67 | L | None | 20/80 | IOL | 253 | 203 | 1 | 20/30 | 240 | −13 |
| 8 | 51 | R | None | 20/100 | −8.50 | 276 | 203 | 2 | 20/20 | 215 | −61 |
| 8 | 51 | L | PDTx2 | 20/400 | −8.75 | 318 | 224 | 2 | 20/30 | 223 | −95 |
| 9 | 47 | L | PDTx2 | 20/200 | IOL | 327 | 210 | 1 | 20/40 | 265 | −62 |
CFT, central foveal thickness; Diff CFT, difference in central foveal thicknesses compared with initial presentation; ID, identification number; INJ, injection(s); IOL, intraocular lens; L, left; NA, not available; PDT, photodynamic therapy; R, right; RE, refractive error; VA, visual acuity.
The CNV imaged on fluorescein angiography was 100% classic without an occult component in all eyes. The mean age at initial treatment was 55.2 (range 38–70) years. Bevacizumab was used as primary treatment in six eyes, and as a rescue treatment in five eyes in which PDT with verteporfin failed to stop leakage of the CNV and which had also experienced a concomitant decline in visual acuity. Eyes were followed‐up for a mean of 153 (range 35–224) days.
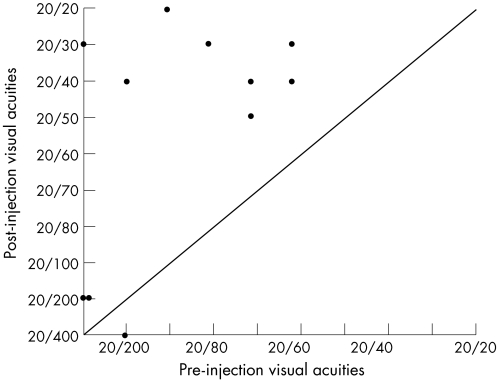
Pre‐injection visual acuity measured 20/50 to 20/100 in six eyes, and 20/200 or worse in five eyes. According to the last follow‐up examination, visual acuity measured 20/20 to 20/40 in seven eyes, 20/50 to 20/100 in one eye and 20/200 or worse in three eyes (fig 1). Three eyes received two injections and eight eyes received one injection. Vision improved by a mean of +3.5 (range −1 to +8) lines, and 8 of 11 eyes achieved 20/50 or better visual acuity. OCT was performed in 10 of 11 eyes before the injection. Central foveal thickness on OCT measured 340 (range 253–664) μm before initial treatment and improved to 234 (range 142–308) μm with intravitreal bevacizumab treatment. This represents an average reduction in absolute central foveal thickness of 103 (range +4 to −356) μm.
Figure 1 Comparison of visual acuities between pre‐injection and post‐injections.
No injection or drug‐related complications, including endophthalmitis, cataract, retinal detachment, glaucoma or uveitis, were observed.
Case report
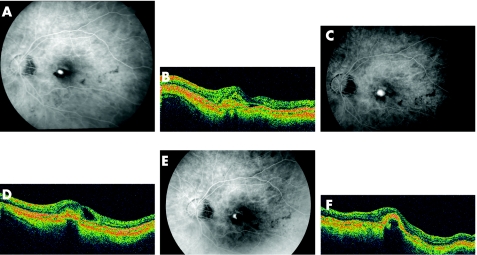
A functionally monocular 47‐year‐old woman with high myopia who had a history of bilateral retinal detachments was referred with decreasing visual acuity measuring 20/100 in her better‐seeing left eye. An actively leaking subfoveal classic CNV without an occult component was visualised on fluorescein angiography, and PDT was performed with a 1400 μm spot (fig 2 A,B). Two months after PDT the visual acuity improved to 20/25, but declined to 20/100 four months after treatment with a recurrent classic subfoveal CNV. Retreatment with a 1400 μm spot was performed without any further benefit. Three months after the second PDT, visual acuity measured 20/200 with an actively leaking CNV (fig 2 C,D). Bevacizumab 1.25 mg intraocular injection was offered to the patient and given without any complication. One month after intravitreal bevacizumab injection, the visual acuity improved to 20/25 with cessation of leakage on fluorescein angiography (fig 2 E,F). Seven months after the initial bevacizumab injection, the visual acuity remains 20/40 without any evidence of an active CNV on fluorescein angiography or OCT. The patient continues to be followed up.
Figure 2 The baseline fluorescein angiogram of the left eye (A) shows an actively leaking, classic subfoveal choroidal neovascularisation (CNV) with a small collection of subretinal fluid on optical coherence tomography (OCT) (B). The visual acuity measured 20/100 and photodynamic therapy (PDT) was performed. After two PDT treatments, the visual acuity declined to 20/200 seven months after presentation with an actively leaking CNV on fluorescein angiogram (C) and an intraretinal, cystic fluid collection on OCT (D). Bevacizumab 1.25 mg was injected into the vitreous cavity with improvement in visual acuity to 20/25 at 1 month. The fluorescein angiogram 1 month after bevacizumab shows staining of the CNV (E) with resolution of the intraretinal fluid on OCT (F) with a dome‐shaped elevation of the retinal pigment epithelial layer from the fibrotic CNV. Four months after intravitreal bevacizumab, the visual acuity remains 20/40 without any change in the OCT (F).
Discussion
The desire for more advanced, efficacious treatments of subfoveal CNV secondary to myopia is based on the natural history of untreated disease. Although the data in the ophthalmic literature are variable, most retrospective studies report a trend towards progressive vision loss. Avila et al's8 study is one of the few studies reporting a positive visual outcome. The authors followed up 70 eyes with untreated myopic CNV for an average of 40.9 months between 1970 and 1982; 50% exhibited either stabilisation or improvement in visual acuity. Hampton et al1 studied 42 eyes at Moorfields Eye Hospital, London, UK, from 1972 to 1982. Visual acuity deteriorated in 71% of eyes, with the overall group experiencing a decline in acuity of 1.6 lines. Tabandeh et al9 published a report of 22 eyes with myopic CNV in individuals ⩾50 years. At 1 year, 5 (23%) eyes showed improvement in visual acuity, with 9 (41%) eyes maintaining stable acuity and 8 (36%) eyes declining. In the longest reported natural history study, Yoshida et al10 followed up 27 Asian eyes with untreated myopic CNV for a minimum of 10 years. At presentation, 8 (29.6%) eyes had an acuity of <20/200, 19 (70.4%) eyes measured 20/40 to 20/200 and 6 (22.2%) eyes measured >20/40. An obvious decline in visual acuity occurred at 3 years, and after 10 years visual acuity declined to ⩽20/200 in 26 (96.3%) eyes, with only 1 (3.7%) eye measuring >20/40.
Treatment options of subfoveal CNV due to pathological myopia include surgical extraction, macular translocation and PDT. Visual acuity results after surgical removal of CNV are variable, making it difficult to ascertain any overall benefit from the procedure.11,12,13,14 Marked expansion of atrophic scar11,12 and high recurrence rates13 are of concern postoperatively. More recently, macular translocation surgery has been evaluated with data limited to a small number of patients and short follow‐up.13,15,16,17,18,19 Argon laser photocoagulation is of limited value and not used as thermal ablation of subfoveal lesions induces severe, immediate, irreversible visual loss. PDT with verteporfin is currently the most widely used treatment for myopic CNV as it enables laser closure of subfoveal CNV without collateral damage to the overlying neurosensory retina. The use of PDT for subfoveal CNV secondary to myopic CNV was validated in the Verteporfin in Photodynamic Therapy Study (VIP). In this prospective, randomised, placebo‐controlled multicentre clinical trial, 72% of PDT‐treated eyes compared with 44% of placebo‐treated patients lost <8 letters on an Early Treatment Diabetic Retinopathy Study chart at 12 months.3 At 2 years, the statistically significant benefit was no longer present, as 36% of verteporfin‐treated eyes and 51% of eyes in the placebo group lost at least 8 letters.4 The combination of intravitreal triamcinolone acetonide with PDT has gained recent popularity over using standard PDT with verteporfin alone as it may decrease the frequency of retreatment and improve visual outcomes. Too few cases have been reported in the literature to conclude whether combination therapy in myopic CNV is a more effective treatment than PDT alone.20,21
Bevacizumab (Avastin), a humanised anti‐VEGF antibody, inhibits VEGF‐A protein and was approved by the US Food and Drug Administration for intravenous use to treat metastatic colorectal cancer in 2004.22 Two recent studies reported the use of intravenous bevacizumab for subfoveal CNV.7,23 Nguyen et al7 described three eyes of two patients treated with a series of four bevacizumab (5 mg/kg) intravenous infusions for CNV secondary to myopia that had failed previous treatments. There was decreased leakage on fluorescein angiography and diminished fluid on OCT in all three eyes, with improvement in visual acuity in two eyes. Fibrosis was present before treatment in the one eye that showed no improvement in visual acuity. Michels et al23 treated nine eyes with intravenous bevacizumab (5 mg/kg) for neovascular AMD. At 12 weeks, mean visual acuity increased by 12 letters, with a clinically relevant reduction in central retinal thickness measured by OCT.
The evolution of the use of bevacizumab for neovascular AMD rapidly shifted from intravenous to intravitreal application in a single case each of neovascular AMD and central retinal vein occlusion with cystoid macular oedema.5,24 In both cases, bevacizumab reduced macular oedema at 4 weeks with improvement in visual acuity in the eye with the vein occlusion. Avery et al6 retrospectively reviewed 81 eyes of 79 patients with subfoveal CNV secondary to AMD treated with monthly bevacizumab injections until macular oedema, subretinal fluid and/or pigment epithelial detachment resolved. In all, 30 of 81 (37%) eyes at 4 weeks and 25 of 51 (49%) eyes at 8 weeks exhibited complete resolution of intraretinal fluid, subretinal fluid and pigment epithelial detachment. Median visual acuity improved from 20/200 to 20/80 at 8 weeks. Side effects related to either bevacizumab or the injection were not observed.
In this limited series of 11 eyes treated with intravitreal bevacizumab for subfoveal CNV secondary to myopic degeneration, 10 eyes showed visual improvement with a reduction in retinal thickness imaged on OCT in 9 of 10 eyes; 3 (27%) eyes required a second injection. Injection and drug‐related complications were not observed.
Currently, there are no published studies evaluating the efficacy or retreatment frequency of intravitreal bevacizumab for myopic subfoveal CNV. The VIP Trial using PDT as the treatment modality is the closest study for comparing the results reported here. No statistically significant visual benefit was observed at 2 years, with a retreatment rate of 91.4% at 3 months and 79% at 6 months. On average, 3.4 treatments were performed at 1 year, with 5.1 treatments performed over a 2‐year period.3,4 Although intravitreal bevacizumab may have a lower retreatment rate than reported for PDT in the VIP Trial, the VIP Trial is a blinded, multicentre study making any direct comparison of PDT with intravitreal bevacizumab for the treatment of myopic CNV impossible.
The results of intravitreal bevacizumab for the treatment of myopic CNV are promising. Long‐term follow‐up with a larger number of patients is essential in further evaluating the safety and efficacy of intravitreal bevacizumab in the treatment of myopic CNV.
Abbreviations
AMD - age‐related macular degeneration
CNV - choroidal neovascularisation
PDT - photodynamic therapy
OCT - optical coherence tomography
VEGF - vascular endothelial growth factor
VIP - Verteporfin in Photodynamic Therapy Study
Footnotes
Competing interests: None declared.
References
- 1.Hampton G R, Kohen D, Bird A C. Visual prognosis of disciform degeneration in myopia. Ophthalmology 198390923–926. [DOI] [PubMed] [Google Scholar]
- 2.Ohno‐Matsui K, Oshida T, Futagami S.et al Patchy atrophy and lacquer cracks predispose to the development of choroidal neovascularization in pathological myopia. Br J Ophthalmol 200387570–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verteporfin in Photodynamic Therapy Study Group Photodynamic therapy of subfoveal choroidal neovascularization in pathologic myopia with verteporfin. 10year results of a randomized clinical trial – VIP report no 1. Ophthalmology 2001108841–852. [DOI] [PubMed] [Google Scholar]
- 4.Verteporfin in Photodynamic Therapy (VIP) Study Group Verteporfin therapy of subfoveal choroidal neovascularization in pathologic myopia: 2‐year results of a randomized clinical trial – VIP report no 3. Ophthalmology 2003110667–673. [DOI] [PubMed] [Google Scholar]
- 5.Rosenfeld P J, Moshfeghi A A, Puliafito C A. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for neovascular age‐related macular degeneration. Ophthalmic Surg Lasers Imaging 200536331–335. [PubMed] [Google Scholar]
- 6.Avery R L, Pieramici D J, Rabana M D.et al Intravitreal bevacizumab (Avastin) for neovascular age‐related macular degeneration. Ophthalmology 2006113363–372. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen Q D, Shah S, Tatlipinar S.et al Bevacizumab suppresses choroidal neovascularization caused by pathological myopia. Br J Ophthalmol. 2005: 89;1368–70, [PMC free article] [PubMed]
- 8.Avila M P, Weiter J J, Jalkh A E.et al Natural history of choroidal neovascularization in degenerative myopia. Ophthalmology 1984911573–1581. [DOI] [PubMed] [Google Scholar]
- 9.Tabandeh H, Flynn H W, Scott I U.et al Visual acuity outcomes of patients 50 years of age and older with high myopia and untreated choroidal neovascularization. Ophthalmology 19991062063–2067. [DOI] [PubMed] [Google Scholar]
- 10.Yoshida T, Ohno‐Matsui K, Yasuzumi K.et al Myopic choroidal neovascularization: a 10‐year follow‐up. Ophthalmology 20031101297–1305. [DOI] [PubMed] [Google Scholar]
- 11.Bottoni F, Perego E, Airaghi P.et al Surgical removal of subfoveal choroidal neovascular membranes in high myopia. Graefe's Arch Clin Exp Ophthalmol 1999237573–582. [DOI] [PubMed] [Google Scholar]
- 12.Ruiz‐Moreno J M, de la Vega C. Surgical removal of subfoveal choroidal neovascularization in highly myopic patients. Br J Ophthalmol 2001851041–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamelin N, Glacet‐Bernard A, Brindeau C.et al Surgical treatment of subfoveal neovascularization in myopia: macular translocation vs. surgical removal. Am J Ophthalmol 2002133530–536. [DOI] [PubMed] [Google Scholar]
- 14.Uemura A, Thomas M A. Subretinal surgery for choroidal neovascularization in patients with high myopia. Arch Ophthalmol 2000118344–350. [DOI] [PubMed] [Google Scholar]
- 15.Glacet‐Bernard A, Simon P, Hamelin N.et al Translocation of the macula for management of subfoveal choroidal neovascularization: comparison of results in age‐related macular degeneration and degenerative myopia. Am J Ophthalmol 200113178–89. [DOI] [PubMed] [Google Scholar]
- 16.Ichibe M, Imai K, Ohta M.et al Foveal translocation with scleral imbrication in patients with myopic neovascular maculopathy. Am J Ophthalmol 2001132164–171. [DOI] [PubMed] [Google Scholar]
- 17.Fujii G Y, Hamayun M S, Pieramici D J.et al Initial experience of inferior limited macular translocation for subfoveal choroidal neovascularization resulting from causes other than age‐related macular degeneration. Am J Ophthalmol 2001131164–171. [DOI] [PubMed] [Google Scholar]
- 18.Tano Y. Pathologic myopia: where are we now? Am J Ophthalmol 2002134645–660. [DOI] [PubMed] [Google Scholar]
- 19.Fujikado T, Ohji M, Kusaka S.et al Visual function after foveal translocation with 360‐degree retinotomy and simultaneous torsional muscle surgery in patients with myopic neovascular maculopathy. Am J Ophthalmol 2001131101–110. [DOI] [PubMed] [Google Scholar]
- 20.Degenring R F, Jonas J B. Photodynamic therapy in combination with intravitreal triamcinolone for myopic choroidal neovascularization. [letter]. Acta Ophthalmol Scand 200583621. [DOI] [PubMed] [Google Scholar]
- 21.Potter M J, Szabo S M, Ho T. Combined photodynamic therapy and intravitreal triamcinolone for the treatment of myopic choroidal neovascularization in a 13‐year old girl. Graefe's Arch Clin Exp Ophthalmol 2005211–3. [DOI] [PubMed] [Google Scholar]
- 22.Hurwitz H, Fehrenbacher L, Novotny W.et al Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. New Engl J Med 20043502335–2342. [DOI] [PubMed] [Google Scholar]
- 23.Michels S, Rosenfeld P J, Puliafito C A.et al Systemic Bevacizumab (Avastin) therapy for neovascular age‐related macular degeneration. Ophthalmology 20051121035–1047. [DOI] [PubMed] [Google Scholar]
- 24.Rosenfeld P J, Fung A E, Puliafito C A. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for macular edema from central retinal vein occlusion. Ophthalmic Surg Lasers Imaging 200536336–339. [PubMed] [Google Scholar]