Abstract
Aim
To assess functional impairment in terms of visual acuity reduction and visual field defects in inactive ocular toxoplasmosis.
Methods
61 patients with known ocular toxoplasmosis in a quiescent state were included in this prospective, cross‐sectional study. A complete ophthalmic examination, retinal photodocumentation and standard automated perimetry (Octopus perimeter, program G2) were performed. Visual acuity was classified on the basis of the World Health Organization definition of visual impairment and blindness: normal (⩾20/25), mild (20/25 to 20/60), moderate (20/60 to 20/400) and severe (<20/400). Visual field damage was correspondingly graded as mild (mean defect <4 dB), moderate (mean defect 4–12 dB) or severe (mean defect >12 dB).
Results
8 (13%) patients presented with bilateral ocular toxoplasmosis. Thus, a total of 69 eyes was evaluated. Visual field damage was encountered in 65 (94%) eyes, whereas only 28 (41%) eyes had reduced visual acuity, showing perimetric findings to be more sensitive in detecting chorioretinal damage (p<0.001). Correlation with the clinical localisation of chorioretinal scars was better for visual field (in 70% of the instances) than for visual acuity (33%). Moderate to severe functional impairment was registered in 65.2% for visual field, and in 27.5% for visual acuity.
Conclusion
In its quiescent stage, ocular toxoplasmosis was associated with permanent visual field defects in >94% of the eyes studied. Hence, standard automated perimetry may better reflect the functional damage encountered by ocular toxoplasmosis than visual acuity.
Toxoplasmosis is probably the most common cause of posterior uveitis in immunocompetent individuals.1 The macula is not infrequently involved,2,3,4,5 and visual acuity may be considerably compromised.4,6,7 If the disease is contracted congenitally, bilateral macular involvement may account for up to 43% of paediatric visual impairment.8,9,10 However, even if macular lesions are larger than one disc area, visual acuity can improve considerably with time,4,11,12 making visual acuity an unreliable measure of the functional damage. Scars involving the extramacular posterior pole, the juxtapapillary region and the optic nerve rarely interfere with visual acuity,13,14,15 but, owing to the transmural destruction of tissue in ocular toxoplasmosis,16 lesions can account for visual field defects in a manner proportional to both the size of the lesion and its proximity to the optic disc.13,17,18,19,20,21 Concomitant vascular occlusion or retinal detachment can further damage the visual field.22 A precipitation of ocular toxoplasmosis in the visual field may thus be expected, but we are aware of only three publications that deal specifically with the impact of ocular toxoplasmosis on the visual field,17,21,23 the first two applying Goldmann kinetic perimetry in active ocular toxoplasmosis, and the most recent one applying the Humphrey automated perimetry to show a relationship between proximity to the optic nerve head and the extension of visual field loss.
In this prospective, cross‐sectional study, we evaluated the prevalence and severity of permanent visual impairment represented in the visual field and in visual acuity in patients with inactive ocular toxoplasmosis. In particular, we assessed the value of automated perimetry in describing the permanent functional damage associated with ocular toxoplasmosis.
Patients and methods
This study drew on a series of 139 consecutive patients with inactive ocular toxoplasmosis. The diagnosis was made according to the clinical picture at presentation with active disease and established diagnostic criteria.
With the approval of the local institutional ethics committee, patients were invited for a free examination at a quiescent inactive stage of their disease including best‐corrected visual acuity (BCVA), applanation tonometry, slit‐lamp inspection and dilated ophthalmoscopy. Colour 50° fundus photographs were taken with a Topcon TRC 50IA fundus camera (FISBA Optics Inc, Switzerland). Photographs were used to compare the location and size of the lesion with the visual field. Standard automated perimetry (program G2, Octopus 101, Haag‐Streit International, Koeniz, Switzerland) was performed with the best‐possible correction.24,25 The G2 program is the Octopus equivalent to the 30‐2 program of the Humphrey visual field analyser. This program uses a higher density of test locations in the macular and perimacular areas (spacing 2.8°). The mean defect is expressed in decibels (dB), the normal range being from −2 to +2 dB. Abnormal values (ie, >2 dB) bear a positive sign.
We defined a visual field scotoma as a cluster of ⩾4 test points, with a deviation of ⩾5 dB from the age‐matched normal value. Integral visual field damage was graded as: mild (mean defect ⩽4 dB), moderate (mean defect 4–12 dB) or severe (mean defect >12 dB). Visual field defects were then compared with the location of lesions from retinal photographs taken on the same day, and categorised morphologically into roughly corresponding and non‐corresponding visual field defects.
For the classification of visual acuity, we referred to the WHO definition of visual impairment (10th revision of the WHO International statistical classification of diseases, injuries and causes of death26), where “low vision” is defined as a BCVA between 20/400 and 20/60 in the better eye. “Blindness” is defined as a BCVA of <3/60 in the better eye. Visual impairment includes low vision and blindness. Correspondingly, we differentiated between the following visual acuity categories: normal (>20/25), mild (20/25 to 20/60), moderately impaired (20/60 to 20/400) and severely (<20/400) impaired.
Statistical evaluation
Descriptive statistics were determined using SPSS, V.13.0. The differences between sets of quantitative data were analysed using Student's two‐tailed t test, with the level of significance being set at p = 0.05.
Results
A total of 61 patients (40 women, 21 men) agreed to participate in the study and were examined. Of these, 8 (13.1%) presented with bilateral involvement. Hence, 69 affected eyes (36 right and 33 left) were included in the analysis. The recurrence‐free period ranged between 2 months and 26 years, with 95% of the eyes having had no recurrence for at least 1 year, and 72% for ⩾2 years. Table 1 shows the results pertaining to patient demographics and disease characteristics, and table 2 the toxoplasmic retinochoroiditis‐related complications.
Table 1 Patient demographics and disease characteristics.
| Parameter | Result, mean (SD) | Range |
|---|---|---|
| Age (years) | 36.2 (13.8) | 13–75 |
| Number of lesions | 3.0 (2.6) | 1–15 |
| Retinal quadrants involved | 1.4 (0.7) | 1–4 |
| BCVA (decimal) | 0.7 (0.4) | 0.01–1.5 |
| Visual field | ||
| Mean defect (dB) | 6.9 (5.4) | −0.8–27.9 |
| Loss variance (dB2) | 38.0 (30.3) | 2.3–152.7 |
BCVA, best‐corrected visual acuity.
Table 2 Eyes with toxoplasmic retinochoroiditis‐related complications.
| n (%) | |
|---|---|
| Total | 15 (21.7) |
| Cataract | 5 (7.2) |
| Macular oedema | 5 (7.2) |
| Macular pucker | 5 (7.2) |
| Retinal detachment | 5 (7.2) |
| Peripheral retinal breaks | 2 (2.9) |
| Branch retinal vein occlusion | 1 (1.4) |
| Branch retinal artery occlusion | 1 (1.4) |
More than one of these complications were encountered in some eyes. Intraocular surgery (cataract extraction, scleral buckling, vitrectomy, peeling) was performed in 11 eyes.
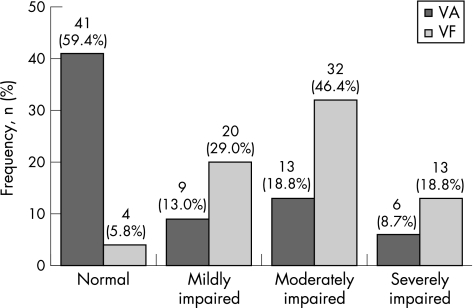
A precipitation of disease in the visual field (ie, mean defect >2 dB) was encountered in 65 of 69 (94.2%) eyes. The topography of visual field defects corresponded well with the morphology of chorioretinal scars in 48 (69.6%) instances. Most of the remaining eyes showed a diffuse or concentric visual field loss as a result of secondary complications (table 2), or manifested multiple chorioretinal scars and/or partial optic nerve atrophy, and/or had undergone surgery. In eyes with a normal or borderline visual field, chorioretinal scars were either extremely discrete or lay very close to the periphery; in this position they did not interfere with the 30° visual acuity. Moderate or severe visual field damage was encountered in 45 of 69 (65.2%) eyes, and moderate or severe reduction of visual acuity in only 19 of 69 (27.5%) eyes, revealing perimetry to measure more accurately the damage induced by ocular toxoplasmosis (p<0.001; table 3 and fig 1). Blindness (visual acuity ⩽20/400; World Health Organization (WHO)26) was encountered in 6 of 69 (8.7%) eyes, and bilaterally impaired visual function in two patients. Normal BCVA was found in 41 (59.4%) eyes, including 33 eyes with a visual acuity of 20/20 or better, while a normal visual field was registered in only four eyes. Taking the categories of normal and mildly impaired function together resulted in 24 (34.8%) eyes meeting the criterion for visual field and 50 (72.5%) eyes meeting that for visual acuity (p<0.001).
Table 3 Grouping of eyes with inactive ocular toxoplasmosis (n = 69) according to visual acuity and the severity of visual field damage.
| VA* | Normal | Mild | Moderate | Severe | MD (SD) (dB) |
|---|---|---|---|---|---|
| VF† | ⩾20/25 | 20/60–20/25 | 20/60–20/400 | ⩽20/400 | |
| Normal to borderline (n = 4) | 4 | 0.97 (1.54) | |||
| Mild visual field damage (n = 20) | 17 | 3 | 2.58 (0.87) | ||
| Moderate visual field damage (n = 32) | 17 | 5 | 8 | 2‡ | 6.61 (1.83) |
| Severe visual field damage (n = 13) | 3 | 1 | 5 | 4 | 16.54 (4.51) |
| Total | 41 | 9 | 13 | 6 | 6.86 (5.39) |
MD, mean defect; VA, visual acuity; VF, visual field.
*According to the WHO classification, all eyes with a VA <20/60 are judged as “visually impaired”, and those with a VA <20/400 as “blind”.
†VF damage was scored as: normal (MD −2 to +2 dB), mild (MD ⩽+4 dB), moderate (MD +4 to +12 dB) or severe (MD >+12 dB), on Octopus standard automated perimetry.
‡Two patients had a severity score for VA below that of VF, 19 were matching with both scores (bold type), and 48 eyes had a more severely affected VF than VA.
Figure 1 Distribution of visual acuity (VA) and visual field (VF) impairment. Categories of VA impairment in ocular toxoplasmosis: normal ⩾20/25; mildly impaired 20/60–20/25; moderately impaired (low vision according to WHO criteria) 20/400–20/60; severely impaired (blind according to WHO criteria) <20/400. Categories of VF damage in ocular toxoplasmosis: normal, with a mean defect that falls within the normal range (−2 to +2 dB). Damage to the VF was scored as: mild (mean defect ⩽+4 dB), moderate (mean defect +4 to +12 dB) or severe (mean defect >+12 dB), on Octopus standard automated perimetry.
Discussion
In the cross‐sectional setting of this study, ocular toxoplasmosis was associated with an abnormal visual field in 94% of our patients' eyes, involving the central 30°. This might not be surprising, but has not been confirmed previously. In 65% of our cases, the visual field loss was moderate to severe. In contrast, reduced visual acuity was encountered in a significantly lower proportion of the cases (40.6%), with 27.5% of the eyes being “visually impaired”.
Only three other groups of investigators have systematically analysed the effect of ocular toxoplasmosis on the visual field.17,21,23 Their results, however, are not directly comparable with ours, as they followed different patients and objectives. Martin et al17 and Schlaegel et al23 used Goldmann kinetic perimetry to measure visual field defects only in peripapillary lesions or in active ocular toxoplasmosis, whereas Stanford et al21 applied Humphrey automated perimetry to show a correlation between the proximity to the optic nerve head and the extension of visual field loss. The findings of these previous studies and those of our own nevertheless confirm that the monitoring of visual acuity alone does not adequately represent the functional damage associated with ocular toxoplasmosis. Even in severe congenital cases of the disease, <50% of the affected eyes exhibit a reduction in visual acuity.4 On the basis of this evidence, it is surprising that visual acuity4 and the number of chorioretinal lesions27 are accepted as measures of the long‐term burden of ocular toxoplasmosis. Clearly, ocular toxoplasmosis is not necessarily associated with profound functional damage,5 and, if it is, then the problem is usually confined to one eye.4,28 However, as ocular toxoplasmosis is functionally represented in the visual field rather than in visual acuity, perimetry might better measure the functional effect of ocular toxoplasmosis and its ocular sequelae, and perhaps be used for monitoring its course. Repeated perimetry would allow the quantification of new functional damage and the detection of clinically unmanifested disease recurrences. It might thus be used as an objective means of quantifying the outcome of prophylactic or treatment strategies.29,30,31
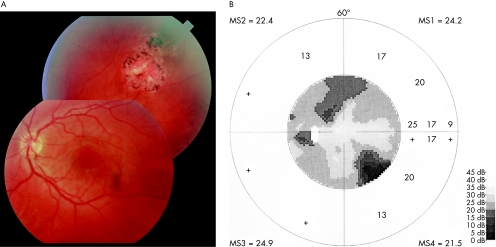
It has to be pointed out that in our series a correlation between size and location of the lesion, and that of the visual field defect was not found in 30% of instances––that is, in 21 eyes, visual field damage was more extensive than would have been expected based on the retinal lesions (fig 2). In all but one case this was attributable to secondary pathologies––that is, optic nerve involvement (4 instances), retinal detachment (4 instances), retinovascular occlusion (2 instances) and non‐toxoplasmic macular pathologies (6 instances). However, poor correlation between lesion and visual field may also be present if a discrete neuroretinal lesion is missed clinically.
Figure 2 Correlation between chorioretinal lesions and visual field defects in ocular toxoplasmosis: a rare example of both good and poor correlation in the same eye; a large chorioretinal scar is found in the superotemporal fundus periphery outside the vessel arcade (A) that corresponds to the deep scotoma in the inferonasal quadrant of the visual field (B). A wedge‐shaped nerve fibre layer defect was traced from the scar to the superior margin of the optic nerve head. A second, shallower scotoma in the Bjerrum area and closer to the blind spot was observed in the superior visual field half. However, no corresponding lesion was found in the central and peripheral retina. Most of the eyes (but not the one depicted) that exhibited a non‐explainable visual field damage (ie, a damage more extensive than it would have been expected based on the retinal lesions) had had secondary complications and/or surgery.
As the aim of our study was to assess the permanent functional impairment associated with chronic ocular toxoplasmosis, only patients in a quiescent disease state were included. This is worth mentioning, as collateral oedema and vitreous involvement do not interfere with the results, whereas in cases with secondary pathologies attributed to ocular toxoplasmosis, such as optic nerve atrophy, the persisting functional effect may differ greatly from the one found early in the disease.32
In conclusion, an effect of ocular toxoplasmosis on the 30° visual field was present in 94% of the eyes in our series. Hence, automated perimetry represents a sensitive means of assessing functional damage associated with ocular toxoplasmosis. We advocate its use as a routine test in the clinical surveillance of patients with ocular toxoplasmosis.
Abbreviations
BCVA - best‐corrected visual acuity
WHO - World Health Organization
Footnotes
Funding: None.
Competing interests: None declared.
References
- 1.Holland G N. Ocular toxoplasmosis: a global reassessment. Part I: Epidemiology and course of disease. Am J Ophthalmol 2003136973–988. [DOI] [PubMed] [Google Scholar]
- 2.Atmaca L S, Simsek T, Batioglu F. Clinical features and prognosis in ocular toxoplasmosis. Japan J Ophthalmol 200448386–391. [DOI] [PubMed] [Google Scholar]
- 3.Bosch‐Driessen L E, Berendschot T T, Ongkosuwito J V.et al Ocular toxoplasmosis: clinical features and prognosis in 154 patients. Ophthalmology 2002109869–878. [DOI] [PubMed] [Google Scholar]
- 4.Mets M B, Holfels E, Boyer K M.et al Eye manifestations of congenital toxoplasmosis. Am J Ophthalmol 1996122309–324. [DOI] [PubMed] [Google Scholar]
- 5.Friedmann C T, Knox D. Variations in recurrent active toxoplasmic retinochoroiditis. Arch Ophthalmol 196981481–493. [DOI] [PubMed] [Google Scholar]
- 6.Kadarisman R S, Marsetio M, Simangunsong L B. Visual impairment and blindness in ocular toxoplasmosis cases. Southeast Asian J Trop Med Public Health 199122(Suppl)99–101. [PubMed] [Google Scholar]
- 7.Brezin A P, Thulliez P, Couvreur J.et al Ophthalmic outcomes after prenatal and postnatal treatment of congenital toxoplasmosis. Am J Ophthalmol 2003135779–784. [DOI] [PubMed] [Google Scholar]
- 8.De Carvalho K M, Minguini N, Moreira Filho D C.et al Characteristics of a pediatric low‐vision population. J Pediatr Ophthalmol Strabismus 199835162–165. [DOI] [PubMed] [Google Scholar]
- 9.Saari M. Toxoplasmic chorioretinitis affecting the macula. Acta Ophthalmol 197755539–547. [DOI] [PubMed] [Google Scholar]
- 10.Suhardjo, Utomo P T, Agni A N. Clinical manifestations of ocular toxoplasmosis in Yogyakarta, Indonesia: a clinical review of 173 cases. Southeast Asian J Trop Med Public Health 200334291–297. [PubMed] [Google Scholar]
- 11.O'Neill J F. The ocular manifestations of congenital infection: a study of the early effect and long‐term outcome of maternally transmitted rubella and toxoplasmosis. Trans Am Ophthalmol Soc 199896813–879. [PMC free article] [PubMed] [Google Scholar]
- 12.Mattews J D, Weiter J J. Outer retinal toxoplasmosis. Ophthalmology 198895941–946. [DOI] [PubMed] [Google Scholar]
- 13.Weiter J J, Wing G L, Trempe C L.et al Visual acuity related to retinal distance from the fovea in macular disease. Ann Ophthalmol 198416174–176. [PubMed] [Google Scholar]
- 14.Banta J T, Davis J L, Lam B L. Presumed toxoplasmosic anterior optic neuropathy. Ocul Immunol Inflamm 200210201–211. [DOI] [PubMed] [Google Scholar]
- 15.Pian D, Ferrucci S, Anderson S F.et al Paramacular coloboma. Optom Vis Sci 200380556–563. [DOI] [PubMed] [Google Scholar]
- 16.Wilder H C. Toxoplasma chorioretinitis in adults. Arch Ophthalmol 195248127–136. [DOI] [PubMed] [Google Scholar]
- 17.Martin W G, Brown G C, Parrish R K.et al Ocular toxoplasmosis and visual field defects. Am J Ophthalmol 19809025–29. [DOI] [PubMed] [Google Scholar]
- 18.Enoch J M, Essock E A, Willilams R A.et al Functional visual effects of lesions located near the optic nerve head. Doc Ophthalmol 198561137–156. [DOI] [PubMed] [Google Scholar]
- 19.Janknecht P, Soriano J M, Funk J.et al Automatische Perimetrie des zentralen Gesichtfeldes bei Erkrankungen der Makula. Klin Monatsbl Augenheilkd 1991199259–263. [DOI] [PubMed] [Google Scholar]
- 20.Wenkel H, Schönherr U. Retinochoroiditis als diagnostischer Hinweis auf eine akute systemische Toxoplasmose bei einem immunkompetenten Patienten. Klin Monatsbl Augenheilkd 1995207314–315. [DOI] [PubMed] [Google Scholar]
- 21.Stanford M R, Tomlin E A, Comyn O. The visual field in ocular toxoplasmosis. Br J Ophthalmol 200589812–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilbert H D. Unusual presentation of acute ocular toxoplasmosis. Graefe's Arch Clin Exp Ophthalmol 198021553–58. [DOI] [PubMed] [Google Scholar]
- 23.Schlaegel T F, Jr, Weber J C. The macula in ocular toxoplasmosis. Arch Ophthalmol 1984102697–698. [DOI] [PubMed] [Google Scholar]
- 24.Weijland A, Fankhauser F, Bebie H.et alAutomated perimetry. Visual field digest. 5th edn. Haag‐Streit, Koeniz, Switzerland 2004: 60–62, 92–106
- 25.Jaakkola A, Vesti E, Immonen I. Correlation between Octopus perimetry and fluorescein angiography after Strontium‐90 plaque brachytherapy for subfoveal exudative age related macular degeneration. Br J Ophthalmol 199882763–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization, WHO Media Centre Magnitude and causes of visual impairment. http://www.who.int/mediacentre/factsheets/fs282/en/index.html
- 27.Holland G N. Ocular toxoplasmosis: a global reassessment. Part II: Disease manifestations and management. Am J Ophthalmol 20041371–17. [PubMed] [Google Scholar]
- 28.Wallon M, Kodjikian L, Binquet C.et al Long‐term ocular prognosis in 327 children with congenital toxoplasmosis. Pediatrics 20041131567–1572. [DOI] [PubMed] [Google Scholar]
- 29.Silveira C, Belfort R, Jr, Muccioli C.et al The effect of long‐term intermittent trimethoprim/sulfamethoxazole treatment on recurrences of toxoplasmic retinochoroiditis. Am J Ophthalmol 200213441–46. [DOI] [PubMed] [Google Scholar]
- 30.Soheilian M, Sadoughi M M, Ghajarnia M.et al Prospective randomized trial of trimethoprim/sulfamethoxazole versus pyrimethamine and sulfadiazine in the treatment of ocular toxoplasmosis. Ophthalmology 20051121876–1882. [DOI] [PubMed] [Google Scholar]
- 31.Holland G N, Lewis K G. An update on current practices in the management of ocular toxoplasmosis. Am J Ophthalmol 2002134102–114. [DOI] [PubMed] [Google Scholar]
- 32.Eckert G U, Melamed J, Menegaz B. Optic nerve changes in ocular toxoplasmosis. Eye Epub 31 Mar 2006 [DOI] [PubMed]