Abstract
Aim
Aim of this study was to investigate the relationship between refractive changes in the eye and biometric changes of the human crystalline lens during accommodation. Furthermore, differences in these relationships between young, healthy emmetropic and myopic subjects were analyzed.
Methods
Mean relative change in anterior chamber depth (ACD), lens thickness (LT), anterior segment length (ASL = ACD + LT) and in objective refraction were simultaneously assessed during near‐point‐induced accommodation in 10 emmetropic and 10 myopic subjects. Via a beam splitter, measurements were performed simultaneously using partial coherence interferometry (PCI) and infrared (IR) photorefraction.
Results
On average, for each diopter of accommodation LT increased by 0.063 mm in emmetropic and by 0.072 mm in myopic eyes, and ACD decreased by 0.047 mm and 0.057 mm, respectively. Mean ASL, indicating the position of the posterior lens pole, increased by 0.009 mm in emmetropic and by 0.013 mm in myopic eyes. The correlation between refractive and biometric changes was found to be essentially linear in both subgroups. Differences in ACD between emmetropic and myopic eyes were statistically significant at an accommodative stimulus of –1 D (p<0.04) and –2 D (p<0.02).
Conclusion
The biometric and refractive changes of the human lens are highly correlated and linear in function in both emmetropic and myopic eyes.
The attempt to restore accommodation in the aging human eye has gained increasing attention in the past decades. This is illustrated by the great interest in accommodating intraocular lenses (IOLs).1 Lens refilling, even though still experimental, seems a promising attempt to restore accommodation. For all these techniques of restoring accommodation, a detailed knowledge of the physiological behaviour of the crystalline lens during ciliary muscle contraction is essential.
Feasibility of such in vivo studies on accommodation is often limited due to the complex physiological mechanisms. Assessing the relationship between all physical properties, such as surface curvatures or the gradient refractive index, and optical properties, such as focal length of the human lens, is possible by an in vitro setting.2 On the other hand, direct biometric in vivo examination of the lens of an accommodating eye is often restricted to animal studies.3,4,5,6,7
Another problem is to find an appropriate way to measure refractive changes during accommodation without influencing the accommodative response due to the study setup. Biometric measurements have frequently been made by utilizing ultrasound, Scheimpflug photography or slit lamp examination.5,6,7,8,9,10,11,12,13,14,15 However, these techniques interfere with the natural viewing conditions. If target driven accommodation is used, the contralateral eye focuses on the optical target while biometry is performed. This can partly lead to convergence movements of the eyes and misalignments between the eye and the measurement system.16 In pharmacological stimulation, such as with pilocarpine, the accommodative changes in young human subjects, as well as in monkeys are slightly different than when elicited with a near‐point target.17,18,19
Hence, the aim of this study was to simultaneously measure the refractive and biometric changes of the anterior eye segment during near‐point induced accommodation in young emmetropic and myopic eyes and to investigate possible differences in the accommodative biometric lens changes between the groups. To our knowledge, this study is the first to attempt to simultaneously measure biometric changes of the lens with partial coherence interferometry (PCI) and measure changes in objective refraction with an infrared photorefractor in the accommodating human eye.
Methods
The study was performed at the Department of Ophthalmology of the Medical University of Vienna, followed the tenets of Helsinki agreement and was approved by the local ethics committee. Written informed consent was obtained from all subjects.
Population
Healthy subjects aged 19 to 31 years were recruited. Subjects were equally divided into two refractive groups: emmetropes with a spherical equivalent (SE) refractive error of less than +/–0.5 D and myopes with a SE refraction of more than –2.5 D (age of onset before 14 years). Age, refraction and gender for each refractive group are shown in table 1.
Table 1 Subjects: gender, age (mean ± SD) and subjectively assessed SE refraction for each refractive group.
| n | sex | age | subj. refraction (mean±SD) | Range (subj. refraction) | |
|---|---|---|---|---|---|
| Emmetropes | 10 | 9f, 1 m | 23.0±2.4 | –0.03 D ±0.08 | –0.25/0 |
| Myopes | 10 | 6f, 4 m | 24.7±3.9 | –4.7 D ±1.5 | –2.75/–7.25 |
All subjects had visual acuities of 1.0 (20/20) or better. Exclusion criteria were astigmatism of >0.5 D, a history of ocular disease, refractive or intraocular surgery, laser treatment, diabetes requiring medical control, glaucoma and retinal pathology. All subjects underwent a detailed ophthalmic examination, including subjective non‐cycloplegic refraction, slit lamp examination and fundoscopy. Values of subjective refraction were verified by objective refraction using the autorefractometer “Refractor 597” (Carl Zeiss Meditec AG, Jena, Germany)(CZM AG).
A beam splitter was used to perform the infrared photorefraction measurements (870 nm) and the PCI measurements (780 nm) simultaneously. Measurements were conducted in a standardized way starting with fixation of a distant target and then proceeding in −1 D steps of defocus, always using the integrated accommodative stimulus of the AC Master. A negative spherical lens moved relatively to an optical target inducing accommodation from 0 up to −5 D. At each level of accommodative stimulus, biometric and refractive measurements were obtained by one examiner who assessed biometric distances during previously started automatic infrared photorefraction under mesopic conditions. Generated mean values of biometric and photorefractive measurements were used for further data evaluation.
Biometric measurements
Biometry of the anterior segment of the fixating eye was assessed with the PCI technique.20,21,22,23,24,25 In this study, a prototype of the commercially available AC Master (CZM AG) was used. It allows defocusing of an internal target (a cross presented on a small LCD screen in the AC Master itself) from 0 up to –5 D, which was used as the stimulus for accommodation. Thus, in order to measure refractive and biometric dynamics in myopes, myopic subjects wore their habitual soft contact lenses for distance correction throughout the experimental trials. A soft contact lens did not influence the quality of the biometric measurements. At least three measurements with a sufficient signal‐to‐noise ratio were obtained at each level of accommodation and mean values were generated.
Anterior chamber depth (ACD) and lens thickness (LT) were measured. Changes in the anterior lens surface position were determined from measurements of ACD, changes in the posterior lens surface position were assessed by adding ACD and LT, resulting in what was named “anterior segment length”(ASL).4
Photorefraction measurements
Change in refraction was measured with an infrared photorefractometer (Powerref II, Plusoptics, Nürnberg, Germany) quantifying refractive error over a range of –8 D to +6 D continuously at 25 Hz from a distance of 1 meter.26,27,28 A high accordance with autorefraction has been reported for this technique.29 Uncontrolled accommodation is one of the main factors interfering with its spherical equivalent assessment in young patients.30 As the assessment of this factor and its differences between emmetropes and myopes was one of the aims of this study, it was not prevented by using cycloplegia, which on the other side would have increased spherical equivalent accuracy.30
The so‐called R‐mode was used assessing the level of refraction and its change over time in continuous, independent measurements within 10 s. Refractive measurements were performed at each level of accommodative stimulus. As the photorefractometer did not provide refraction values of all single measurements in between the 10 s, a minimum of 50 single consecutive measurements were defined mandatory to use the mean value (provided by the photorefractometer itself) for further data evaluation.
Data analysis
For further data evaluation, we used mean values of at least 50 refractive measurements and of at least three biometric measurements at each level of accommodation. Differences in change in refraction and lens dimension were statistically analyzed with the paired t‐test, where p<0.05 was the significance level.
Results
There were no significant differences between objectively and subjectively assessed refraction values in both groups.
Relative changes in objective refraction for each diopter of accommodative stimulus are shown in table 2.
Table 2 Relative changes in objective refraction for each diopter of accommodative stimulus for each refractive group (mean ± SD).
| accommodative stimulus | relative change in objective refraction (in D) | |
|---|---|---|
| emmetropes | myopes | |
| –1 D | –0.42±0.31 | –0.43±0.24 |
| –2 D | –1.00±0.42 | –1.61±1.08 |
| –3 D | –2.22±1.03 | –2.36±1.01 |
| –4 D | –3.07±0.70 | –3.40±1.33 |
| –5 D | –4.61±0.84 | –4.19±1.53 |
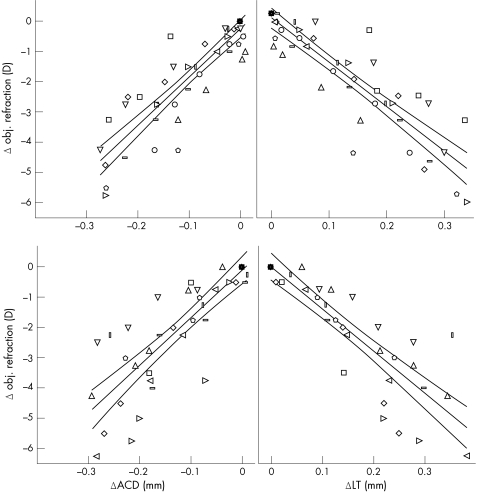
The individual biometric responses of the lens found during accommodation are shown in fig 1 for 10 emmetropic eyes (upper) and for 10 myopic eyes (lower). The biometric changes were linearly correlated to the refractive changes in the emmetropic (ACD:r2 = 0.81; LT:r2 = 0.81) and in the myopic eyes (ACD:r2 = 0.72; LT:r2 = 0.77).
Figure 1 Relative change in ACD and LT (mm) versus relative change in objective refraction (D) during accommodation for 10 emmetropic eyes (upper) and 10 myopic eyes (lower). The regression line including 95% confidence interval is depicted. Each symbol represents the correlation between accommodative and biometric changes in one patient depending on the accommodative stimulus.
LT increased on average by 0.057 mm ±0.008 in emmetropic and 0.060 mm ±0.010 in myopic eyes for each diopter of accommodative stimulus. However, comparing change in objective refraction with increasing LT we found a difference between the groups. In the emmetropic eyes, on an average LT increased by 0.063 mm and ACD decreased by 0.047 mm per diopter of increasing refraction. The resulting functions for the relative change in refraction for emmetropic eyes are
Δ refraction = –0.14–13.58× Δ LT
Δ refraction = –0.25+15.97× Δ ACD
with “Δrefraction” meaning relative change in objective refraction in D, “Δ LT” relative change in LT in mm and “Δ ACD” relative change in ACD in mm.
In myopic eyes, on an average LT increased by 0.072 mm and ACD decreased by 0.057 mm per diopter of increasing refraction. The resulting functions for change in refraction for myopic eyes are
Δ refraction = 0.00–13.93× Δ LT
Δ refraction = –0.10+15.72× Δ ACD
(abbreviations as explained above).
Mean absolute values of ACD, LT and ASL at an accommodative stimulus of 0 D up to –5 D are shown in table 3 for emmetropic and myopic eyes.
Table 3 Absolute values (mean ± SD) in mm of anterior chamber depth (ACD), lens thickness (LT) and anterior segment length (ASL) at an accommodative stimulus level of 0 up to –5 D in 10 emmetropic and 10 myopic eyes.
| emmetropes | myopes | |||||
|---|---|---|---|---|---|---|
| ACD | LT | ASL | ACD | LT | ASL | |
| 0 D | 3.795±0.167 | 3.598±0.230 | 7.393±0.231 | 4.127±0.178 | 3.661±0.133 | 7.789±0.216 |
| –1 D | 3.787±0.156 | 3.622±0.222 | 7.410±0.225 | 4.094±0.174 | 3.687±0.128 | 7.739±0.114 |
| –2 D | 3.747±0.138 | 3.665±0.222 | 7.412±0.230 | 4.072±0.178 | 3.747±0.138 | 7.777±0.094 |
| –3 D | 3.673±0.148 | 3.738±0.228 | 7.436±0.239 | 3.987±0.182 | 3.831±0.149 | 7.818±0.212 |
| –4 D | 3.608±0.153 | 3.830±0.248 | 7.439±0.250 | 3.912±0.183 | 3.889±0.121 | 7.814±0.233 |
| –5 D | 3.524±0.127 | 3.954±0.199 | 7.474±0.264 | 3.881±0.189 | 3.959±0.143 | 7.841±0.234 |
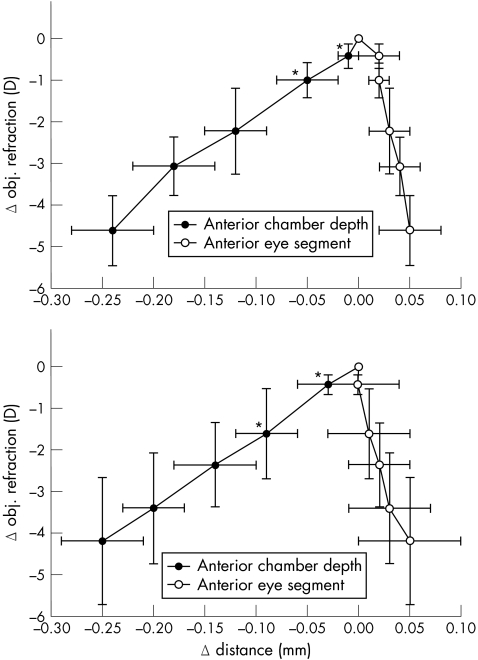
The relative change of the anterior lens surface position compared to that of the posterior pole is shown in fig 2 for the emmetropic and the myopic eyes, respectively. In emmetropic eyes, the lens change was primarily induced by a change in posterior pole position during accommodation from 0 to –1 D (see fig 2). In the myopic eyes, the lens change was primarily caused by a change in the anterior lens pole during accommodation from 0 to –2 D. Accordingly, there was a significant difference in ACD between emmetropic and myopic eyes at an accommodative stimulus of –1 D (p<0.04) and –2 D (p<0.02). Differences in posterior lens surface positions between emmetropic and myopic eyes did not reach statistical significance at an accommodation of 1 D (p = 0.24) or 2 D (p = 0.27), neither did changes in LT (1 D: p = 0.24, 2 D: p = 0.15). Percentages of movement of the anterior and the posterior lens pole in emmetropic and myopic eyes during accommodation and p values of the differences in anterior lens surface position are shown in table 4.
Figure 2 Mean relative change in anterior (ACD) and posterior (ASL) lens surface position versus mean relative change in objective refraction in 10 emmetropic eyes (upper) and 10 myopic eyes (lower) during accommodation for an accommodative stimulus of 0 D to –5 D. Error bars indicate standard deviations, asterisks indicate statistically significant differences in anterior lens surface position between emmetropes and myopes.
Table 4 Percentages of movement compared to the 0 D position of the anterior and the posterior lens pole in emmetropic and myopic eyes during accommodation for an accommodative stimulus of 0 to –5 D.
| Emmetropes ant./post. surface (%) | Myopes ant./post. surface (%) | P (ant. surface emmetropes/myopes) | |
|---|---|---|---|
| –1 D | 32/68 | 94/6 | 0.04 |
| –2 D | 71/29 | 95/5 | 0.02 |
| –3 D | 81/19 | 89/11 | 0.31 |
| –4 D | 80/20 | 86/14 | 0.22 |
| –5 D | 83/17 | 83/17 | 0.62 |
P‐values refer to the differences in anterior lens surface position between emmetropic and myopic eyes.
Between emmetropes and myopes, there was neither a significant difference in change in objective refraction at each level of defocus (–1 D: p = 0.93; –2 D: p = 0.16; –3 D: p = 0.8; –4 D: p = 0.59; –5 D: p = 0.61), nor in accommodative amplitude (emmetropes: 4.61 D±0.84; myopes: 4.19 D±1.53; p = 0.53).
To examine intraobserver reproducibility, two emmetropic and two myopic subjects were investigated on a second study day using the identical protocol. In all four subjects mean SD between individually assessed values were 0.028 mm for changes in ACD, 0.02 mm for changes in LT and 0.26 D for changes in objective refraction. Hence, there was a high intraobserver reproducibility.
Discussion
One interesting finding of this study was that there seems to be a slight difference in the initial biometric changes of the lens with accommodation between emmetropes and myopes. Furthermore, a linear relationship between refractive and biometric changes of the human eye during accommodation could be shown. This study is based on a previous study published by Drexler and coworkers, who investigated lenticular changes during target driven accommodation, and provides new information, as additionaly refractive changes were assessed simultaneously.20
The differences in accommodative changes between both groups, the emmetropic and the myopic, were distinct at an accommodative stimulus of –1 D and –2 D (see table 3). In the emmetropic group, there was an initial posterior shift at the posterior lens pole up to about 1 D of accommodation. With increasing accommodative effort, lens thickening was then dominated by changes of the anterior lens surface as expected. However, in the myopic group, the lens thickening was initially induced by a forward shift of the anterior lens surface with an essentially stationary posterior pole. Accordingly, there was a statistically significant difference in ACD between emmetropic and myopic eyes at an accommodative stimulus of –1 D and –2 D.
These results indicate that there are differences in the accommodative changes of the lens between emmetropes and myopes, which have not been described previously. In a chicken eye model Choh showed that the lenticular accommodative apparatus is affected by ametropia.31 Inducing myopia in chicken eyes for 7 days led to statistically significantly shorter focal lengths of the crystalline lenses and smaller accommodative amplitudes. Hence, even if this was observed in an animal study, it could suggest that myopia can have effects not only on eye growth, but also on the accommodative biometric changes of the human crystalline lens. These effects on accommodative mechanisms could be due to differences in zonule fibre insertion or in the relative position of the ciliary muscle to the lens equator.32 However, as only changes in LT and ACD and in refraction were assessed this reasoning is entirely hypothetical, and it has to be emphasized that the sample size in both groups was rather small.
In comparison to other biometry techniques such as ultrasound, slitlamp pachymetry or Scheimpflug photography, this study's test setup showed several advantages. PCI is a very precise no‐contact method for ocular biometric measurements with a high precision (<10 μm) and resolution (∼12 μm).20,21,22,23,24,25 During a measurement, the examined eye fixates on an optical target, hence convergence eye movements due to presentation of the target to the contralateral eye can be avoided.16 Furthermore, accommodation is stimulated with negative trial lenses, a principle reported to result in even more consistent and reliable results than pilocarpine stimulation.18,33 On the other hand, differences in accommodative response to minus‐lens‐induced blur between emmetropes and myopes have also been reported previously.34
Vilupuru and coworkers, who investigated Edinger Westphal stimulated accommodation in rhesus monkeys, found results that were similar to those of this study.4 Measurements were performed dynamically, however, not simultaneously, but in 2 sessions, once refraction and once biometry, with the help of the ultrasound technique. Interestingly, the values for mean increase in LT per diopter accommodated in rhesus monkeys are identical to ours for emmetropic human subjects (0.063 mm/D). In monkeys, ACD decreased on average by 0.046 mm/D compared to 0.047 mm/D in our emmetropic group. They also found the relationship between refractive and biometric changes during accommodation to be linear, which is in good standing with our results.
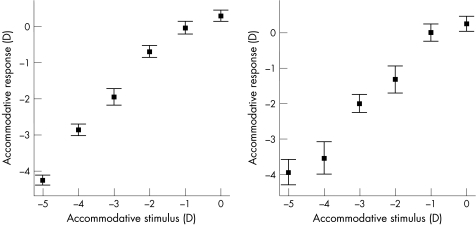
In a previous study published by Drexler and coworkers correlations between biometric and refractive changes during accommodation were not found to be as linear as in this study.20 But Drexler investigated biometric changes of the anterior eye segment for the whole accommodative amplitude and accommodation was induced by offering a moveable fixation target, and not by presetting negative spherical lenses. Values of mean change in ACD and LT per D were smaller than in this study. This could be in part because biometric changes were measured as function of stimulus amplitude and not accommodative response altitude, as it was performed in this study. To visualize the difference between these two methods, the differences between accommodative stimulus and accommodative response of this study's subgroups are shown in fig 3.
Figure 3 Correlation between accommodative stimulus (AC Master) and objective accommodative response (photorefractometer) in emmetropes (left) and myopes (right) (mean ± SE).
Until now it has not been cleared to what extent the posterior lens pole position changes during accommodation. Helmholtz supposed the posterior lens pole position to remain stable during accommodation, Koretz and coworkers found similar results using Scheimpflug technique in humans and using ultrasound in monkeys.35,36 Even recently, Strenk and coworkers reported that the posterior lens pole does not move during accommodation using magnetic resonance imaging.37 As opposed to this, other authors measured a change in posterior lens pole position during accommodation in monkeys and in humans.4,17,20 Results of this study provide evidence that there is a definite backward movement of the posterior lens surface also in humans. Furthermore, differences in the extent of movement were found between the emmetropic and the myopic group at different levels of defocus (see fig 2) that did not reach statistical significance.
To conclude, even if accommodative refractive changes in the eye are primarily due to changes in lens surface curvature, it is important to know that there are linear relationships between refractive and axial biometric changes of the human anterior eye segment during accommodation. As the study population was small, it is not clear in how far accommodative differences between emmetropes and myopes need to be considered for the development of new techniques for restoring accommodation such as accommodative IOLs and lens refilling.
Abbreviations
ACD - anterior chamber depth
ASL - anterior segment length
IOLs - intraocular lenses
LT - lens thickness
PCI - partial coherence interferometry
SE - spherical equivalent
Footnotes
Competing interests: WD is a consultant to Carl Zeiss Meditec AG. No other author has a proprietary or financial interest in any material or method mentioned.
References
- 1.Findl O. Intraocular lenses for restoring accommodation: hope and reality. J Refract Surg 200521321–323. [DOI] [PubMed] [Google Scholar]
- 2.Glasser A, Campbell M C. Biometric, optical and physical changes in the isolated human crystalline lens with age in relation to presbyopia. Vision Res 1999391991–2015. [DOI] [PubMed] [Google Scholar]
- 3.Vilupuru A S, Glasser A. Dynamic accommodation in rhesus monkeys. Vision Res 200242125–141. [DOI] [PubMed] [Google Scholar]
- 4.Vilupuru A S, Glasser A. Dynamic accommodative changes in rhesus monkey eyes assessed with A‐scan ultrasound biometry. Optom Vis Sci 200380383–394. [DOI] [PubMed] [Google Scholar]
- 5.Koretz J F, Bertasso A M, Neider M W.et al Slit‐lamp studies of the rhesus monkey eye: III. The zones of discontinuity. Exp Eye Res 198846871–880. [DOI] [PubMed] [Google Scholar]
- 6.Koretz J F, Bertasso A M, Neider M W.et al Slit‐lamp studies of the rhesus monkey eye: II. Changes in crystalline lens shape, thickness and position during accommodation and aging. Exp Eye Res 198745317–326. [DOI] [PubMed] [Google Scholar]
- 7.Koretz J F, Neider M W, Kaufman P L.et al Slit‐lamp studies of the rhesus monkey eye. I. Survey of the anterior segment. Exp Eye Res 198744307–318. [DOI] [PubMed] [Google Scholar]
- 8.Beers A P, Van Der Heijde G L. In vivo determination of the biomechanical properties of the component elements of the accommodation mechanism. Vision Res 1994342897–2905. [DOI] [PubMed] [Google Scholar]
- 9.van der Heijde G L, Beers A P, Dubbelman M. Microfluctuations of steady‐state accommodation measured with ultrasonography. Ophthalmic Physiol Opt 199616216–221. [DOI] [PubMed] [Google Scholar]
- 10.Zadnik K, Mutti D O, Adams A J. The repeatability of measurement of the ocular components. Invest Ophthalmol Vis Sci 1992332325–2333. [PubMed] [Google Scholar]
- 11.Shum P J, Ko L S, Ng C L.et al A biometric study of ocular changes during accommodation. Am J Ophthalmol 199311576–81. [DOI] [PubMed] [Google Scholar]
- 12.Beers A P, van der Heijde G L. Age‐related changes in the accommodation mechanism. Optom Vis Sci 199673235–242. [DOI] [PubMed] [Google Scholar]
- 13.Beers A P, van der Heijde G L. Analysis of accommodation function with ultrasonography. Doc Ophthalmol 1996921–10. [DOI] [PubMed] [Google Scholar]
- 14.Dubbelman M, Van der Heijde G L, Weeber H A.et al Changes in the internal structure of the human crystalline lens with age and accommodation. Vision Res 2003432363–2375. [DOI] [PubMed] [Google Scholar]
- 15.Dubbelman M, Van der Heijde G L, Weeber H A. Change in shape of the aging human crystalline lens with accommodation. Vision Res 200545117–132. [DOI] [PubMed] [Google Scholar]
- 16.Ciuffreda K, Kruger P. Dynamics of human voluntary accommodation. Am J Ophthalmol 198865365–370. [DOI] [PubMed] [Google Scholar]
- 17.Ostrin L A, Glasser A. Comparisons between pharmacologically and Edinger‐Westphal‐stimulated accommodation in rhesus monkeys. Invest Ophthalmol Vis Sci 200546609–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wold J E, Hu A, Chen S.et al Subjective and objective measurement of human accommodative amplitude. J Cataract Refract Surg 2003291878–1888. [DOI] [PubMed] [Google Scholar]
- 19.Koeppl C, Findl O, Kriechbaum K.et al Comparison of pilocarpine‐induced and stimulus‐driven accommodation in phakic eyes. Exp Eye Res 200580795–800. [DOI] [PubMed] [Google Scholar]
- 20.Drexler W, Baumgartner A, Findl O.et al Biometric investigation of changes in the anterior eye segment during accommodation. Vision Res 1997372789–2800. [DOI] [PubMed] [Google Scholar]
- 21.Findl O, Drexler W, Menapace R.et al High precision biometry of pseudophakic eyes using partial coherence interferometry. J Cataract Refract Surg 1998241087–1093. [DOI] [PubMed] [Google Scholar]
- 22.Drexler W, Baumgartner A, Findl O.et al Submicrometer precision biometry of the anterior segment of the human eye. Invest Ophthalmol Vis Sci 1997381304–1313. [PubMed] [Google Scholar]
- 23.Drexler W, Findl O, Menapace R.et al Partial coherence interferometry: a novel approach to biometry in cataract surgery. Am J Ophthalmol 1998126524–534. [DOI] [PubMed] [Google Scholar]
- 24.Hitzenberger C K, Baumgartner A, Drexler W.et al Interferometric measurement of corneal thickness with micrometer precision. Am J Ophthalmol 1994118468–476. [DOI] [PubMed] [Google Scholar]
- 25.Fercher A F, Hitzenberger C K, Drexler W.et al In vivo optical coherence tomography. Am J Ophthalmol 1993116113–114. [DOI] [PubMed] [Google Scholar]
- 26.Glasser A, Kaufman P L. The mechanism of accommodation in primates. Ophthalmology 1999106863–872. [DOI] [PubMed] [Google Scholar]
- 27.Schaeffel F, Wilhelm H, Zrenner E. Inter‐individual variability in the dynamics of natural accommodation in humans: relation to age and refractive errors. J Physiol 1993461301–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schaeffel F, Farkas L, Howland H. Infrared photoretinoscope. Appl Opt 1987261505–1509. [DOI] [PubMed] [Google Scholar]
- 29.Allen P M, Radhakrishnan H, O'Leary D J. Repeatability and validity of the PowerRefractor and the Nidek AR600‐A in an adult population with healthy eyes. Optom Vis Sci 200380245–251. [DOI] [PubMed] [Google Scholar]
- 30.Schimitzek T, Lagreze W A. Accuracy of a new photo‐refractometer in young and adult patients. Graefes Arch Clin Exp Ophthalmol 2005243637–645. [DOI] [PubMed] [Google Scholar]
- 31.Choh V, Sivak J G. Lenticular accommodation in relation to ametropia: the chick model. J Vis 20055165–176. [DOI] [PubMed] [Google Scholar]
- 32.Oliveira C, Tello C, Liebmann J M.et al Ciliary body thickness increases with increasing axial myopia. Am J Ophthalmol 2005140324–325. [DOI] [PubMed] [Google Scholar]
- 33.Ostrin L A, Glasser A. Accommodation measurements in a prepresbyopic and presbyopic population. J Cataract Refract Surg 2004301435–1444. [DOI] [PubMed] [Google Scholar]
- 34.Gwiazda J, Thorn F, Bauer J.et al Myopic children show insufficient accommodative response to blur. Invest Ophthalmol Vis Sci 199334690–694. [PubMed] [Google Scholar]
- 35.von Helmholtz H. Ueber die Akkommodation des Auges. A. v. Graefes Arch Klin Exp Ophthalmol 18551–74.
- 36.Koretz J F, Cook C A, Kaufman P L. Accommodation and presbyopia in the human eye. Changes in the anterior segment and crystalline lens with focus. Invest Ophthalmol Vis Sci 199738569–578. [PubMed] [Google Scholar]
- 37.Strenk S A, Strenk L M, Koretz J F. The mechanism of presbyopia. Prog Retin Eye Res 200524379–393. [DOI] [PubMed] [Google Scholar]