Abstract
Aim
To review the clinical and histopathological features, treatment and outcomes of squamous cell carcinoma of the eyelids.
Methods
76 patients with eyelid squamous cell carcinoma treated in an oncology referral hospital between 1997 and 2006 were reviewed retrospectively. Age, sex, risk factors, duration of symptoms, size and location of lesion, previous recurrences, presence of perineural invasion (PNI) and orbital invasion, histological subtype, inflammatory response of peritumoral tissue were recorded and analysed.
Results
Mean (SD) lesion size was 2.4 (0.36) mm. 27 (35.5%) cases were previously recurrent. The most common histological subtype was well differentiated (59.2%). The rates of PNI and orbital invasion were 23.8% and 43.4%, respectively. 63 patients underwent surgery, whereas others were treated with external radiotherapy or chemotherapy. Recurrence or presence of residual tumour rate was 22.4%, most of them had orbital invasion. Regional lymph node metastasis was detected in 5 (6.6%) cases.
Conclusions
Advanced deep local invasion was not rare in this study, as a result of treatment delay and previous inadequate treatments. Adverse prognostic factors associated with secondary orbital invasion are previous recurrences, longer duration of lesion, larger lesion size, and presence of PNI. Well‐differentiated subtype and strong inflammatory response are good prognostic factors.
Squamous cell carcinoma (SCC) is the second most common malignancy of the eyelid skin.1 In the eyelid, although it occurs much less commonly than basal cell carcinoma, it is more aggressive and invasive than basal cell carcinoma. If treatment is delayed, periocular SCC can metastasise and invade the orbital and intracranial structure, hence it has a considerable potential of mortality and morbidity.2
We conducted a retrospective study to review the clinical, histopathological and treatment features, and the outcome of eyelid SCCs that was seen in an oncology referral hospital over a period of 9.5 years.
Methods
One of the authors' medical records of 76 patients treated as eyelid squamous cell carcinoma, from 1997 to 2006, were reviewed retrospectively. Invasive SCCs are included only if confirmed histopathologically and derived from eyelids and periorbital area. Patients who refused all treatment alternatives or were lost to follow‐up just after treatment were excluded.
A detailed history was taken for each patient; information on symptoms, duration of lesion, previous treatment and risk factors was recorded. Some patients were referred to our centre after being diagnosed with SCC by incisional biopsy, whereas others were referred owing to a recurrent tumour. Other patients were diagnosed by an incisional or excisional biopsy in our centre. Although most of the patients were treated with surgical excision, some were treated with external radiotherapy and/or chemotherapy.
Surgical excision was performed on all patients except for 13. Frozen‐section examination of surgical margins was performed in five recurrent cases to maintain clear margins. In others, 4–5 mm safety margins from clinical borders of the lesion was used. Small defects were closed directly. Large defects were closed with antibiotic ointment and dressing, while waiting for histological confirmation of tumour clearance by paraffin section. Further excision was made from the positive tumour margins, until clear margins were reached. After histopathological analysis was completed (3–7 days), reconstruction was performed by various oculoplastic techniques.
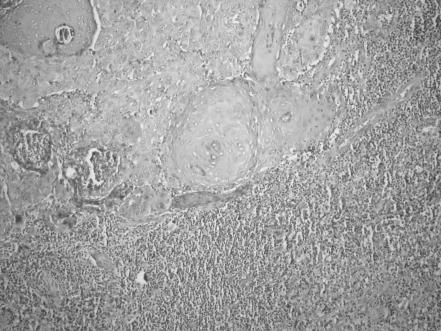
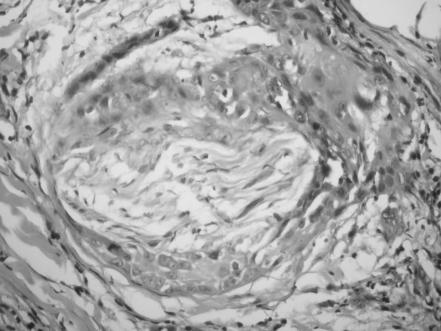
In all excision material, histopathological criteria are clearance of surgical margins, degree of differentiation, presence of perineural infiltration (PNI), inflammatory response around the tumour tissue and actinic keratosis adjacent to tumour. Specimens were stained with haematoxylin and eosin to observe the perineural spread and peritumorous infiltration of inflammatory cells. The tumours were also graded, well, moderately or poorly differentiated, according to the degree of differentiation. All tumours were histologically analysed with regard to presence of peritumorous lymphocytic infiltration, and the intensity of infiltration was scored as 0, 1+, 2+ and 3+ (fig 1).
Figure 1 Peritumorous lymphocytic infiltration (score 3+; haematoxylin and eosin, ×200).
After planning this study, all patients were invited to the clinic by letter or telephone communication. If the patient did not respond to the invitation, follow‐up time was detected according to the last visit of the patient.
Results
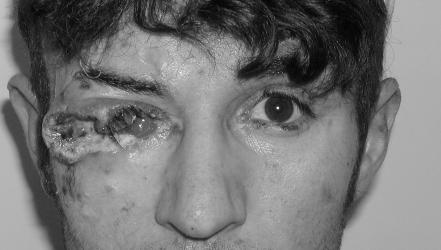
Of the 76 patients, 41 (53.9%) were male and 35 (46%) were female. Patients' ages at the time of presentation ranged from 11 to 93 years (mean (SD) 64.26 (3.54), median 67 years). Three patients were <30 years old, one was 21 years old with epidermodysplasia verruciformis (fig 2), and two were 11 and 27 years old with xseroderma pigmentosum. Most patients were >50 years old.
Figure 2 A large eyelid squamous cell carcinoma that invaded the orbit in a patient with epidermodysplasia verricuformis. Informed patient consent was obtained for publication of this figure.
In all, 47 (61.8%) patients had chronic exposure to the sun according to history. Actinic solar keratosis was detected clinically in 32 patients. On histological examination, a neighbouring region of actinic keratosis was detected around the excised tumorous tissue, in 22 of 63 (34.9%) cases.
The time between the onset of lesion and presentation ranged from 2 to 60 months (mean (SD) 13.50 (2.62), median 9 months). In all, 27 (35.5%) patients had recurrent eyelid SCC, previously treated elsewhere. These previous treatments were surgery in 19 cases, cryotheraphy in 2 cases and radiotherapy in 6.
The site of the lesion was defined as the area in which the lesion first appeared. The most common site of the lesions was the lower lid (37 (48.9%) cases). The sites of the lesion were medial canthus (n = 14), upper lid (n = 9), lateral canthus (n = 6) and periorbital areas such as cheeks, nose and forehead (then extended to eyelids; n = 10).
The size of the lesions ranged from 5 to 65 mm (mean (SD) 2.4 (0.36), median 2.25 mm) maximum diameter. According to the lesion size, we classified patients to three different groups, as:
group A, <1 cm (n = 21);
group B, >1 cm and no orbital invasion (n = 22); and
group C, >1 cm with orbital invasion (n = 33).
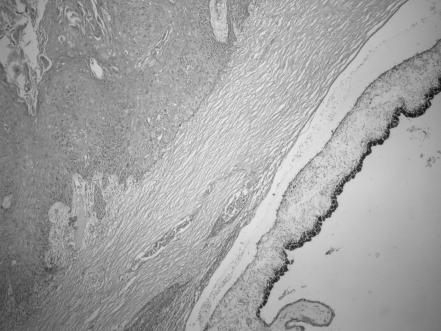
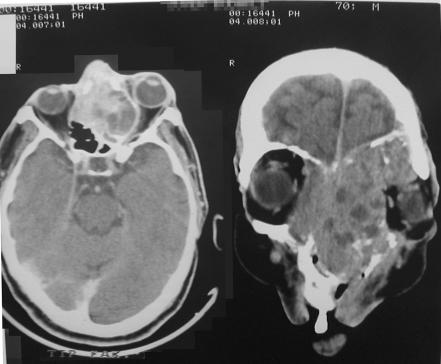
Orbital invasion was detected by clinical, radiological and histological findings. Paranasal sinus involvement was present in seven patients with orbital invasion. Scleral invasion was detected in four patients (fig 3). Excessive local spread was developed as cavernous sinus, nasopharynx and contralateral orbital involvement in one patient (fig 4), and intracranial involvement in another, during the follow‐up. Preauricular lymph node metastasis was detected in five patients at presentation; cervical lymph node metastasis occurred later in one.
Figure 3 Invasion of sclera by squamous cell carcinoma (haematoxylin and eosin, ×200).
Figure 4 Computed tomography of an advanced stage of a recurrent eyelid squamous cell carcinoma that extended into paranasal sinuses and controlateral orbit.
Sixty three patients underwent wide surgical excision including exenteration. In all, 13 (20.6%) of these received postoperative radiotherapy owing to positive tumour surgical margins and/or detection of perineural infiltration. Fourteen patients underwent exenteration. Combined bone resection in three patients, sinusectomy in four patients were performed. External radiotherapy was applied as the first choice of treatment for palliation in five patients who were not suitable for surgery (because of patients' refusal or systemic handicap). For similar reasons, chemotherapy was applied in six patients. Simultaneous radiotherapy and chemotherapy was used in two patients. Table 1 shows the treatment for each group of patients, and recurrence or residual tumours for each treatment modality. In metastatic cases, three underwent lymphadenectomy and radical neck dissection, and in one, additional radiotherapy was applied to the neck.
Table 1 Number of patients, recurrences and residual tumours for each group according to treatment modalities.
| Group A | Group B | Group C | |
|---|---|---|---|
| Excision | 21/0 | 22/1 | 0 |
| Excision–RT | 0 | 0 | 5/0 |
| Excision–CT | 0 | 0 | 1/0 |
| Exenteration | 0 | 0 | 6/0 |
| Exenteration–RT | 0 | 0 | 4/1 |
| Exenteration‐sinusectomy–RT | 0 | 0 | 4/2 |
| RT | 0 | 0 | 5/5 |
| CT | 0 | 0 | 6/6 |
| RT–CT | 0 | 0 | 2/2 |
CT, chemotherapy; RT, radiotherapy.
Group A, patients with lesion <1 cm; group B, patients with lesion >1 cm and no orbital invasion; and group C, patients with lesion >1 cm with orbital invasion.
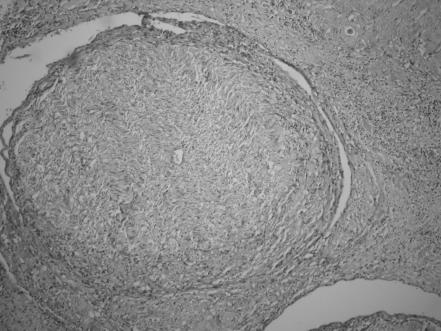
Microscopic PNI was analysed only in 63 patients who underwent excisional surgery. Although, in two cases, PNI was detected inside the incisional biopsy material by chance, it is thought to be unreliable to decide on the absence of PNI by examining only a small part of tumour tissue from patients treated without surgery. For this reason, statistical analysis between PNI and other findings was performed by using the data from patients who underwent excisional surgery. PNI was detected microscopically in 15 of 63 (23.8%) patients (figs 5, 6). Nine of them had orbital invasion and six were recurrent cases. Pain was the most frequent symptom of PNI (seven cases, all had orbital invasion); sensory disturbances such as loss of sensation or formication was described by five patients. Extraocular motility disturbances were frequent in patients with advanced orbital invasion owing to mass effect, five patients had ocular motor palsies, in two of whom PNI was detected histologically and in the remaining three, surgery was not performed.
Figure 5 Perineural invasion by squamous cell carcinoma (haematoxylin and eosin, ×400).
Figure 6 Optic nerve invasion by squamous cell carcinoma (haematoxylin and eosin, ×400).
Table 2 summarises the previous recurrences, PNI, metastasis, histological subtypes and grade of perilesionary inflammation for each group of patients.
Table 2 Previous recurrences, perineural invasion, metastasis, histological subtypes and grade of perilesionary inflammatory response for each group.
| Group A (n = 21) | Group B (n = 22) | Group C (n = 33) | |
|---|---|---|---|
| SCC histology | |||
| Well differentiated | 18 | 15 | 12 |
| Moderately differentiated | 3 | 7 | 12 |
| Poorly differentiated | 0 | 0 | 9 |
| Primary | 21 | 20 | 8 |
| Previously recurrent | 0 | 2 | 25 |
| PNI | 2 | 4 | 9 |
| Metastasis | 0 | 0 | 5 |
| Perilesionary inflammation | |||
| No inflammation | 3 | 0 | 5 |
| Grade 1 | 2 | 3 | 14 |
| Grade 2 | 10 | 9 | 12 |
| Grade 3 | 6 | 9 | 2 |
PNI, perineural invasion; SSC, squamous cell carcinoma.
Group A, patients with lesion <1 cm; group B, patients with lesion >1 cm and no orbital invasion; and group C, patients with lesion >1 cm with orbital invasion.
Statistical analysis showed that duration of lesion was strongly correlated with lesion size (Pearson's correlation test, p<0.001), presence of orbital invasion and PNI (independent t test, p<0.001). We found a significant difference between primary and recurrent tumours according to presence of orbital invasion (χ2 test, p = 0.002) and lesion size (t test, p<0.001). But the difference was not significant for PNI (χ2 test, p = 0.09). Presence of orbital invasion is correlated with that of PNI (χ2 test, p = 0.002) and lesion size (t test, p<0.001).
Well‐differentiated histology was the most common subtype (n = 45, 59.2%). In all, 22 (28.9%) tumours were moderately differentiated and 9 (11.8%) tumours were poorly differentiated. All tumours with poorly differentiated histology were in group C. The degree of differentiation was in a reverse correlation with orbital invasion (Mann–Whitney U test, p<0.001) and lesion size (Spearman's correlation test, p<0.001, r = −0.393), and in an insignificant association with PNI (Mann–Whitney U test, p = 0.3).
The inflammatory response around the tumour was weaker in larger lesions (Spearman's correlation test, p = 0.007, r = −0.306), in poorly differentiated histology (Spearman's correlation test, p<0.01, r = 0.334), in cases with orbital invasion (Mann–Whitney U test, p<0.001) and with PNI (Mann–Whitney U test, p = 0.11).
Recurrences occurred only in one patient in group B, and no recurrence was observed in group A. Two patients died owing to systemic problems in group B. In group C, in all patients who were treated only with radiotherapy and/or chemotherapy, a decrease in lesion size was observed, but not a disease‐free state. Six patients died because of tumour‐related reasons and 10 patients died from other causes, mostly because of old age. In group C, rate of the recurrence or residual tumour was 48.5% (16 cases). The total recurrence rate was 22.4% (17 cases).
The mean (SD) follow‐up time was 24.75 (2.78) months (median; 21.0 months) with a range of 3–55 months.
Discussion
SCC of the eyelid and periocular region typically has no pathognomonic feature that allows its differentiation from other lesions.3 As the clinical appearance of eyelid SCC has a broad spectrum and may resemble various benign and malignant lesions,4 we preferred all patients who had lesions >1 cm and had undergone a histological diagnosis before treatment. Numerous studies had confirmed the inaccuracy of preoperative clinical diagnosis, even by experienced observers.5,6,7,8 Donaldson et al4 reported that 62.7% of the lesions were correctly identified as squamoproliferative lesions preoperatively.
SCC of the eyelids is a more rapidly growing and aggressive neoplasm than basal cell carcinoma. Early diagnosis and adequate treatment of the eyelid SCC is very important, because of its ability to invade the orbital and intracranial regions and metastasise to the lymph nodes and distant organs. In this study, the time interval between the onset of the lesion and presentation to our clinic ranged from 2 to 60 months. Commonly, a delay in presentation for treatment was obviously present. Some patients preferred to neglect their eyelid lesion until disturbing symptoms such as haemorrhage, pain or eyelid function abnormalities occurred. On the other hand, 27 patients had recurrent lesions that were treated previously elsewhere. Statistical analysis showed that longer duration of lesion and delay in treatment causes an increase in lesion size, and the risk of orbital invasion and PNI. Similarly, analysis about recurrences showed similar results. It is well known that recurrences and delay in medical care worsen the prognosis of the disease.9 In this study, owing to these reasons, the rate of recurrences is very high, especially in group C. Continued efforts at public education regarding the benefits of early diagnosis and treatment are the answer to the problem.9
SCC is by far the most common of the secondary epithelial neoplasms in the orbit.10 Today, most cases of SCC are diagnosed when small, and cured with surgical excision. However, once orbital invasion develops, orbital exenteration is needed to achieve the cure.9 To our knowledge, the rate of orbital invasion by eyelid SCC in this study (43.4%) is the highest among all those reported in the literature. Several factors may account for this. Firstly, more advanced disease is higher at our centre than at other settings. Although smaller eyelid SCCs are treated without any problem all over the country, advanced and recurrent tumours are referred to our centre. Secondly, the proportion of elderly patients from rural areas, who come from low socioeconomic levels, and the lack of health consciousness is higher among our patients, because of the general public hospital characteristic of our centre.
Despite the fact that 13 patients did not undergo surgery and PNI was not explored histologically in these cases, it was detected in 15 of 63 (23.8%) patients. Most of these patients had advanced orbital tumours. We estimated that in patients treated with treatment modes other than surgery, the incidence of PNI might be high because of the advanced and aggressive course of their disease. The rate of PNI is relatively high compared with others in the literature; however, the clinical and histopathological characteristics of this phenomenon are the subject of another study by us. Perineural spread of cutaneous SCC is associated with an increased risk of local recurrence and distant metastasis, but may also be the direct cause of death when the primary tumour on the head and face gains access to the intracranial cavity via the cranial nerves.11,12,13 Several factors have been found to correlate with the increased death and poor prognosis for cutaneous SCC. These include prior treatments, lesion size >2 cm, increased depth, poor histopathological differentiation and immunosuppression.14 However, McNab et al11 reported that the presence of perineural involvement was the most effective factor that increases the rates of recurrence and metastasis.
Human solid tumours are often infiltrated by mononuclear inflammatory cells. The infiltration of lymphoid cells in carcinomas has often been interpreted as an indication of an active immune response to the tumour and, thus, a better prognostic sign. The infiltration by lymphocytes, especially T lymphocytes has been associated with tumour size, stage and patients' survival in a variety of human cancers including stomach, gallbladder and colorectal carcinomas.15,16,17,18 The histological detection of a peritumorous lymphocytic infiltration in patients with SSC of the larynx, pharynx or oral cavity is inversely correlated with the development of cervical lymph node metastases and is therefore a favourable prognostic factor.19 In our study, we showed that the presence of intensive peritumorous lymphocytic infiltration in SSCs of eyelids is a favourable prognostic factor too.
The rate of regional lymph node metastasis in this study was 6.6% (5 patients). The previously reported incidences of regional lymph node metastasis associated with eyelid SSCs reported previously have varied widely, from 10% to 21.4%, in different retrospective series. Faustina et al20 indicated that the overall rate of regional lymph node metastasis in patients with SCC of periocular skin might be as high as 24%. Regional lymph nodes are generally believed to be the most common first site of metastasis of eyelid SCC.3,14,21 The rate of regional metastasis is surprisingly low compared with the rate of deep local invasion in this study. This can be explained with the relatively short follow‐up time of patients. Some patients with advanced local disease, lost to follow‐up, and probable regional or distant metastasis remained undiagnosed. Another explanation of this low metastasis rate is that, despite the high rate of the advanced disease, we histologically showed that actinic keratosis accompanied 34.9% of lesions. It is well known that SCCs arisen from actinic keratosis have a very low risk of metastasis.2
A handicap for this study is that although it was conducted over a long time interval, some patients were lost to follow–up, and a long follow‐up time was not reached owing to lack of patient compliance.
In conclusion, this study has the one of the largest series of eyelid SCC that includes commonly advanced cases. Early detection of lesions can markedly reduce ocular morbidity, secondary orbital invasion and recurrences. Among the factors that cause poor prognosis are low degree of differentiation, weaker peritumourous inflammatory response, previous recurrences, larger lesion size, and PNI. Delay in treatment and recurrences are the most important preventable factors. Patients with eyelid SCC should be advised to attend regular follow‐up examinations. Public education about eyelid tumours and its prevention methods would minimise the mortality and morbidity of the disease.
Abbreviations
PNI - perineural invasion
SSC - squamous cell carcinoma
Footnotes
Competing interests: None declared.
References
- 1.Malhatra R, Huilgol S C, Huynh N T.et al The Australian Mohs database. Periocular squamous cell carcinoma. Ophthalmology 2004111617–623. [DOI] [PubMed] [Google Scholar]
- 2.Reifler D M, Hornblass A. Squamous cell carcinoma of the eyelid. Surv Ophthalmol 198630349–365. [DOI] [PubMed] [Google Scholar]
- 3.Cook B E, Jr, Bartley G B. Treatment options and future prospects for the management of eyelid malignancies: an evidence based update. Ophthalmology 20011082088–2100. [DOI] [PubMed] [Google Scholar]
- 4.Donaldson M J, Sullivan T J, Whitehead K J.et al Squamous cell carcinoma of the eyelids. Br J Ophthalmol 2002861161–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nixon R L, Dorevitch A P, Marks R. Squamous cell carcinoma of the skin: accuracy of clinical diagnosis and outcome of follow up in Australia. Med J Aust 1986144235–236. [PubMed] [Google Scholar]
- 6.Kersten R C, Ewing Chow D, Kulwin D R.et al Accuracy of clinical diagnosis of cutaneous eyelid lesions. Ophthalmology 1997104479–484. [DOI] [PubMed] [Google Scholar]
- 7.Cook B E, Bartley G B. Epidemiologic characteristics and clinical course of patients with malignant eyelid tumors in an incidence cohort in Olmsted County, Minnesota. Ophthalmology 1999106746–750. [DOI] [PubMed] [Google Scholar]
- 8.Kwitko M L, Boniuk M, Zimmerman L E. Eyelid tumors with reference to lesions confused with squamous cell carcinoma. I. Incidence and errors in diagnosis. Arch Ophthalmol 196369693–697. [DOI] [PubMed] [Google Scholar]
- 9.Howard G R, Nerad J A, Carter K D.et al Clinical characteristics associated with orbital invasion of cutaneous basal cell and squamous cell tumors of the eyelid. Am J Ophthalmol 1992113123–133. [DOI] [PubMed] [Google Scholar]
- 10.Johnson T E, Tabbara K F, Weatherhead R G.et al Secondary squamous cell carcinoma of the orbit. Arch Ophthlmol 199711575–78. [DOI] [PubMed] [Google Scholar]
- 11.McNab A A, Francis I C, Benger R.et al Perineural spread of cutaneous squamous cell carcinoma via the orbit. Ophthalmology 19971041457–1462. [DOI] [PubMed] [Google Scholar]
- 12.Wilcsek G A, Francis I C, Egan C A.et al Superior oblique palsy in a patient with a history of perineural spread from periorbital squamous cell carcinoma. J Neuroophthalmol 200020240–241. [PubMed] [Google Scholar]
- 13.Veness M J, Biankin S. Perineural spread leading to orbital invasion from skin cancer. Aust Radiol 200044296–302. [DOI] [PubMed] [Google Scholar]
- 14.Rowe D E, Caroll R J, Day C L. Prognostic factors for local recurrences, metastasis and survival rates in squamous cell carcinoma of the skin, ear and lip. J Am Acad Dermatol 199226976–990. [DOI] [PubMed] [Google Scholar]
- 15.Songun I, Vande Velde C J, Arrends J W.et al Classification of gastric carcinoma using the Goseki system provides prognostic information. Cancer 1999852114–2118. [DOI] [PubMed] [Google Scholar]
- 16.Nakakubo Y, Miyamoto M, Cho Y.et al Clinical significance of immune cell infiltration within gallbladder cancer. Br J Cancer 2003891736–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menon A G, Janssen‐van Rhijn C M, Morreau H.et al Immune system and prognosis in colorectal cancer : a detailed immunohistochemical analysis. Lab Invest 200484493–501. [DOI] [PubMed] [Google Scholar]
- 18.Tachibana T, Onodera H, Tsuruyama T.et al Increased intratumor Valpha24‐positive natural killer T cells a prognostic factor for primary colorectal carcinomas. Clin Cancer Res 2005117322–7327. [DOI] [PubMed] [Google Scholar]
- 19.Badoual C, Hans S, Rodriguez J.et al Prognostic value of tumor‐infiltrating CD4+ T‐cell subpopulations in head and neck cancers. Clin Cancer Res 200612465–472. [DOI] [PubMed] [Google Scholar]
- 20.Faustina M, Diba R, Ahmadi M A.et al Patterns of regional and distant metastasis in patients with eyelid and periocular carcinoma. Ophthalmology 20041111930–1932. [DOI] [PubMed] [Google Scholar]
- 21.McCord C D, Jr, Cavanagh H D. Microscopic features and biologic behaviour of eyelid tumors. Ophthalmic Surg 198011671–681. [PubMed] [Google Scholar]