Abstract
Aim
To quantify the rate of recurrence of acute anterior uveitis (AAU), and evaluate the influence of associated risk factors.
Methods
We retrospectively reviewed the case notes of 185 patients with acute anterior uveitis, from their time of presentation to August 2001. The time to the first three recurrences of AAU from the onset of the disease was recorded, as well as the site of recurrence. Information regarding risk factors (for example (HLA‐B27) status, spondyloarthropathy (SpA), family history of AAU/SpA and history of non‐specific joint pain) were also collected.
Results
Patients were followed up until their third relapse, or up to the censoring date (August 2001) if less than three relapses had occurred. The median length of follow‐up was 35 months. One hundred and twenty‐two patients (66%) developed at least one relapse and 67 (36%) had three or more relapses. Kaplan‐Meier estimate of median interval between disease onset and the first relapse was 24 months 95% CI (16 to 34) and between the first and second relapse was 14 months 95% CI (9 to 22), and was 15 months 95% CI (10 to 25) months between the second and third relapse. Using Cox regression only the number of previous relapses was significantly associated with the risk of AAU recurrence. There was no significant association between other reported risk factors and the risk of relapse, and neither did any risk factor significantly modify the association between previous relapses and AAU recurrence (p>0.066 for all interactions). There was a borderline significant difference in survival according to the laterality pattern of recurrences (ipsilateral, alternate, or bilateral) with a slightly greater than expected number of events in those with bilateral recurrence (p = 0.048).
Conclusion
Patients with previous relapse(s) of AAU have a greater risk of AAU recurrence compared to those at disease onset but the risk of recurrence appears not to increase in a dose‐response manner with increasing number of previous relapses. Demographic and extraocular features do not appear to influence the rate, or risk of recurrence of AAU.
Acute anterior uveitis (AAU) is the most common type of uveitis in Northern Europe and most associated with recurrence.1 AAU is associated with the (HLA‐B27) gene and the spondyloarthropathies (SpA) which include ankylosing spondylitis, inflammatory bowel disease, reactive arthritis, psoriatic arthritis and possibly Behcet's disease. These extraocular diseases can follow a chronic relapsing and remitting course, but the association between them and acute recurrences of AAU has not been adequately explained.
Previous work on risk factors has focused on SpA and/or HLA‐B27 status and their influence on uveitis severity. Some authors found that HLA‐B27 positive disease, especially if associated with SpA, to be associated with more complications and worse visual outcome, compared with those who are HLA‐B27 negative.2 However, others have not been able to confirm these findings,3 and some have found more complications in the HLA‐B27 negative group.4
There is a great range in the reported number and rate of recurrences of AAU. Tay Kearney et al found one patient with 26 attacks and one with an interval of 35 years to the first recurrence.5 Some authorities recommend that patients with an uncomplicated first attack of AAU require no further investigation than examination and history alone.6 This presumes that patients with recurrence are more likely to yield a systemic diagnosis but this has never been demonstrated. However given there is such a wide variation in time to recurrence of disease, patients should be given full information about the indications for rheumatological referral at their first attack of AAU. The necessity to investigate for SpA in the absence of systemic symptoms is questionable.
An accurate estimate of the risk of recurrence requires long‐term cohort studies, as the recurrence rate from cross‐sectional studies will vary depending on follow up. The purpose of this study was to estimate the risk of recurrence in a large cohort of patients with AAU from a primary referral centre using time‐to‐event analysis. We also analysed whether certain risk factors (HLA‐B27 status, SpA, family history of AAU or SpA, and history of non‐specific joint pain) were associated with an increased risk of single or multiple recurrences.
Methods
Subjects
We reviewed the notes of 185 consecutive patients with AAU presenting from January 1997 to August 2001. These included both incident cases presenting for the first time as well as patients first presenting prior to January 1997 and returning with recurrent attacks (prevalent cases). All were primary referrals to a rural district general hospital with a dedicated uveitis clinic set up in January 1997. All cases had sudden onset, painful attacks lasting less than 4 months, upon which a diagnosis of AAU was made. The majority of patients had uncomplicated disease and were treated with the standard tapering regimen of topical steroids and mydriatics. Patients were followed up 4–6 weeks later. When collecting relapse data, only patients who had been in remission in the preceding 4 weeks were included. The patient database is registered with the local Caldicott data protection guardian.
Age at presentation and gender were noted. The time to the first three recurrences of AAU was recorded, and the follow‐up time calculated. The site of recurrence was recorded; unilateral for attacks which always occurred in the same eye; unilateral alternating for recurrences which occurred in either eye, or simultaneously bilateral.
Data concerning the risk factors such as HLA‐B27 status, SpA, family history of AAU/SpA and non‐specific arthritis were collected. HLA‐B27 testing and sacroiliac imaging were not performed universally. A diagnosis of definite SpA or unclassified arthropathy was made after patients with a suggestive history were referred to a rheumatologist. HLA‐B27 status and the presence of uveitis were not used as additional diagnostic criteria in contrast to the European Spondyloarthropathy Study Group definitions.7
Statistical analysis
Time‐to‐event analysis was performed using STATA version 9. Cox‐proportional hazards regression was used to assess association with risk factors and Kaplan‐Meier survival curves were used to summarize survival. Subjects contributed follow up time in months from date of entry into the study (ie, disease onset) until their first three relapses or until the censoring date (August 2001) if less than three relapses had occurred. To accommodate multiple recurrences per subject, the relapses were assumed to be ordered in the sense that subjects at risk for a firstt relapse should have had diagnosis of disease, and those at risk of a second relapse had to have experienced 1 relapse and those at risk of third relapse had to have experienced two relapses. The conditional risk set formulation of the Cox model was used to derive the hazard function and possible non‐independence of the multiple relapse times within individuals was accounted for using Stata's “cluster” option to obtain robust standard errors for the hazard ratios in the fitted Cox regression model.8 Further information about the statistical methods and data setup can be found in supporting supplemental information.
There was no significant difference in the results when confined to incident cases presenting since January 1997 so all incident and prevalent cases were included in the results.
Results
Subjects
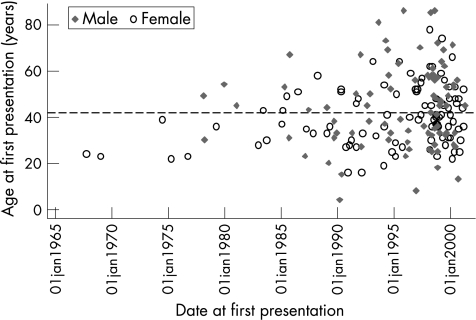
Figure 1 shows a plot of age at presentation versus calendar year of recruitment for the 185 patients in the study by sex. Patients were recruited continuously since October 1967 to August 2001 with just over half (n = 102/185, 55%) of the patients recruited as incident cases (ie, after January 1997). Ninety‐eight (53%) patients were male and eighty‐seven were female (47%). The mean age at onset of AAU was 39.8 years (median 39, (16–78)) for males and 45.0 years (median 45, (4–86)) for females. The median length of follow‐up was 35 months (3–259). One hundred and sixty‐seven patients (90%) were followed up for 12 months or more, 88 (48%) for 3 years or more, 58 (31%) for 5 years or more, and 22 (12%) for 10 years or more.
Figure 1 Graph showing age at presentation of patients versus calendar year of recruitment
Survival times
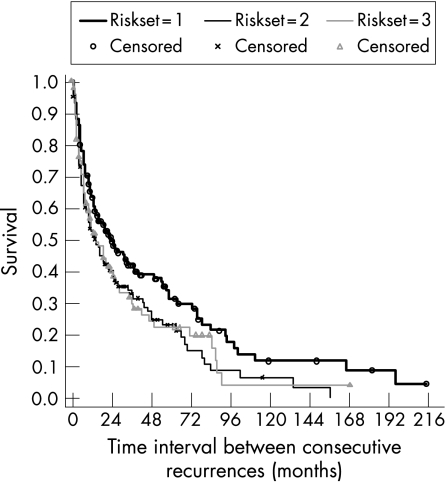
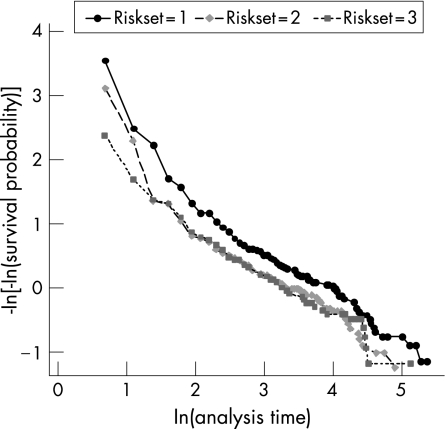
Table 1 shows a summary of the Kaplan‐Meier survival estimates of time intervals between consecutive recurrences of AAU, by risk set, and an overall estimate calculated for all risk sets combined representing time from previous disease episode to the next recurrence. Figure 2 shows the corresponding Kaplan‐Meier survival curve, including the complimentary log‐log plot for assessing validity of proportional hazard assumption by risk set (fig 3). In risk set 1, all 185 study subjects were at risk of one relapse but 63 (34%) were censored before relapsing and the remaining 122 (66%) relapsed. The median time to first relapse was 24 months, with total time at risk of 5358 person months giving a recurrence rate of 2.28 per 100 person months. The 122 who relapsed in risk set 1 were at risk of a second relapse in risk set 2, of which 27 (22%) of them were censored and 95 (78%) had a second relapse. The median time to second relapse was 14 months, with total time at risk since first relapse of 2854 person‐months, giving an incidence rate of 3.33 per 100 person months. Finally, the 95 who relapsed in set 2 were at risk of having a third relapse in risk set 3, of which 28 (29%) were censored and 67 (71%) had a third relapse. The median time from second to third relapse was 15 months and the incidence rate was 3.24 per 100 person months. The proportional hazard assumption was satisfied by the risk set variable as suggested by the approximately parallel lines for the three groups (fig 2) and also confirmed by a non significant chi‐square test based on Schoenfeld residuals (χ2 = 0.02, p = 0.99). Therefore the Cox regression models were adjusted for risk set and Stata's cluster option was used to correct standard errors for multiple relapses per patient.
Table 1 Kaplan‐Meier estimates of time interval in months between consecutive recurrences of AAU and estimated recurrence rates.
| Risk set | No. of subjects | Censored N (Row %) | Relapsed N (Row %) | 25% recurrence time (95% CI) | 50% recurrence time (95% CI) | 75% survival time (95% CI) | Person‐months (pmths) at risk | Recurrence rate per 100 pmths (95%CI) |
|---|---|---|---|---|---|---|---|---|
| 1 | 185 | 63 (34%) | 122 (66%) | 7 (5 to 10) | 24 (16 to 34) | 76 (59 to 98) | 5358 | 2.28 (1.81 to 2.84) |
| 2 | 122 | 27 (22%) | 95 (78%) | 4 (4 to 6) | 14 (9 to 22) | 48 (36 to 70) | 2854 | 3.33 (2.58 to 4.26) |
| 3 | 95 | 28 (29%) | 67 (71%) | 5 (3 to 7) | 15 (10 to 25) | 47 (28 to 87) | 2066 | 3.24 (2.34 to 4.43) |
| Total* | 402 | 118 | 284 | 6 (5 to 7) | 19 (14 to 24) | 55 (50 to 79) | 10278 | 2.76 (2.34 to 3.26) |
*The recurrence time statistics on the total row represent the expected time since previous episode to the next relapse (ie, either from disease onset to 1st relapse for those in risk set 1, or from first to second relapse for those in risk set 2, or from second to third relapse for those in risk set 3) calculated using all 402 data records on 185 subjects with the standard errors adjusted to account for multiple relapses per subject.
Figure 2 Kaplan‐Meier survival estimates of time interval between consecutive recurrences of AAU by risk set.
Figure 3 Test of proprtional hazards assumption by risk set.
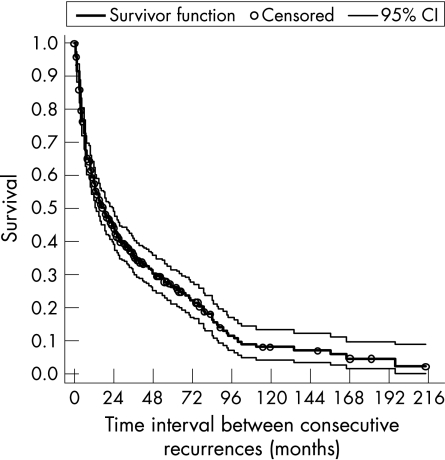
The overall estimate of median time from previous episode to the next relapse, taking account for multiple relapses per subject, was 19 months 95% CI (14 to 24) months and the incidence rate was estimated as 2.76 95% CI (2.34 to 3.26) per 100 person months (table 1). The overall survival curve (fig 4) suggested that on average 90% 95% CI (84% to 94%) of patients with AAU would relapse within 8.3 years of follow‐up (ie, survival probability <0.10 after 100 months).
Figure 4 Overall Kaplan‐Meier survival estimate.
Laterality of recurrence
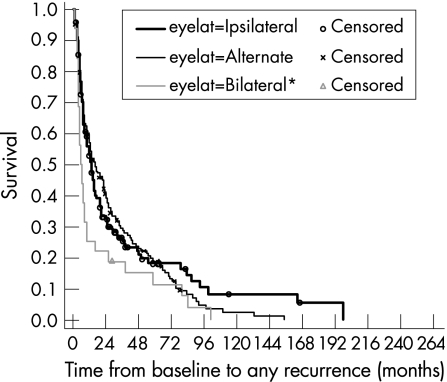
Of the 122 subjects who had at least one relapse, 57 (47%) had unilateral attacks, 52 (43%) had unilateral‐alternating attacks, and 11 (9%) had bilateral attacks. Information on the nature of attack was unavailable for two subjects. Figure 5 shows the survival curves by laterality of recurrence. A log‐rank test suggested a borderline significant difference in the survival times for the three types of recurrence patterns (p = 0.048), showing slightly greater than expected number of events in those with bilateral recurrence had there been no difference.
Figure 5 Kaplan‐Meier survival estimates, by recurrence pattern.
Influence of ‘risk factors'
Table 2 shows results from the Cox regression model fitted to assess influence of risk factors on the risk of recurrence. Of the SpA group, only data regarding AS was of sufficient size to perform statistical analysis; two patients had psoriatic arthritis, one had reactive arthritis and two patients had an enteropathic arthropathy. Only the number of relapses was significantly associated with the risk of AAU recurrence, suggesting that compared to those at disease onset (risk set 1), the risk of AAU recurrence was 40% higher in those with one previous relapse (risk set 2) and was 34% higher in those with two previous relapses (risk set 3). Thus the risk of recurrence appeared not to increase in a dose‐response manner. None of the other risk factors were significantly associated with risk of recurrence, but the point estimates for some of the relative risks were moderately large with wide 95% confidence intervals suggesting that these might be significant with larger series of patients. A test of interactions did not suggest that any of the risk factors significantly modified the association of previous relapses and risk of AAU recurrence (p>0.066 for all interactions).
Table 2 Univariate* associations between ‘risk factors' and risk of AAU recurrence in a Cox regression model allowing for multiple events per patient.
| Variable | Relapses within risk factor category | Cox‐regression results | |||
|---|---|---|---|---|---|
| No Relapse n (Row%) | Relapse n (Row%) | Total | RR (95% CI) | p | |
| Number of previous relapses | |||||
| None (risk set 1) | 63 | 122 | 185 | 1 | |
| 1 relapse (risk set 2) | 27 | 95 | 122 | 1.40 (1.09, 1.80) | 0.008 |
| 2 relapses (risk set 3) | 28 | 67 | 95 | 1.34 (1.00, 1.80) | 0.046 |
| Age (per year) | – | – | 185 | 1.00 (0.99, 1.01) | 0.649 |
| Sex | |||||
| Female | 30 (34.5%) | 57 (65.5%) | 87 | 1 | |
| Male | 33 (33.7%) | 65 (66.3%) | 98 | 0.93 (0.72 to 1.21) | 0.606 |
| HLA B27 status | |||||
| Positive | 25 (35.7%) | 45 (64.3%) | 70 | 1 | |
| Negative | 7 (30.4%) | 16 (69.6%) | 23 | 1.40 (0.92 to 2.13) | 0.116 |
| AS | 0.169 | ||||
| Not AS | 40 (35.4%) | 73 (64.6%) | 113 | 1 | |
| Radiological sacroiliitis | 17 (32.1%) | 36 (67.9%) | 53 | 1.18 (0.89 to 1.57) | 0.243 |
| History of sacroiliitis (normal X‐ray) | 6 (31.6%) | 13 (68.4%) | 19 | 1.26 (0.79 to 2.02) | 0.335 |
| Unclassified arthralgia | |||||
| No | 50 (33.6%) | 99 (66.4%) | 149 | 1 | |
| Yes | 13 (36.1%) | 23 (63.9%) | 36 | 1.28 (0.87 to 1.87) | 0.206 |
| Family history AAU/SpA | |||||
| Negative | 58 (35.2%) | 107 (64.8%) | 165 | 1 | |
| Positive | 5 (25%) | 15 (75%) | 20 | 1.02 (0.59 to 1.74) | 0.952 |
*Each univariate association was adjusted for the significant effect of number of previous relapses. Test of interactions did not suggest that any of the risk factors significantly modified the association of previous relapses and risk of acute anterior uveitis (AAU) recurrence (p>0.066 for all interactions).
Discussion
Acute anterior uveitis is a frequent reason for attending emergency clinics. Lack of knowledge about the etiology of the disease, the precipitants of relapse, and means to prevent them can lead to frustration for patients. Accurate epidemiological information about recurrence may allow patients to have a better understanding, as well give a clearer insight into disease pathogenesis. No clear risk factor has been found to influence the course and frequency of attacks of AAU. Much of the discrepancy has been due to the use of cross‐sectional studies and populations including chronic attacks or posterior inflammation.
This is the first study using survival analysis and adds to the previous cross sectional series. Using a primary referral population we have avoided the bias in series from referral centers with patients suffering more severe arthritis or AAU. The major findings were that, with sufficient follow up, multiple recurrences appear to be by far the most frequent outcome, with the number of previous relapses being significantly associated with an increase risk of AAU recurrence. Previous studies found that patients suffered a mean of three attacks as would be expected with a mean follow up of 4–10 years, and the number of attacks was from one to more than 20. Tay‐Kearney et al examined 148 HLA B27 positive patients and found a mean of three attacks in those followed up for more than a year.5 The range was 1–26 attacks and the median interval was 14 months (range 1–420 months). Other series of AAU patients have found an interval of 12–30 months.4,9
We also found the presence of HLA‐B27 and SpA did not appear to increase the risk of, or time to, recurrence. This contrasts with studies that have found the presence of HLA‐B27, extraocular inflammatory disease and demographic variables to be of significance. Norn found the overall recurrence rate to be 55% and all with arthritis had a recurrence.10 Others found that the presence of SpA with HLA‐B27 was associated with higher relapse rates than those who were HLA‐B27 positive alone, or HLA‐B27 negative. The recurrence rate in HLA‐B27 patients with SpA was 74% (mean of 5.2 attacks), compared with 28% in those HLA‐B27 negative (mean 1.4 attacks).2 However the follow up was only 15–19 months, included patients with painless and chronic anterior uveitis and a blindness rate of 7% suggesting bias towards serious ocular disease. These results were partly supported by the results of Rothova,3 who could not confirm a difference in severity in HLA‐B27 positive patients with or without ankylosing spondylitis. In contrast Linssen et al found no increase of recurrence in those with HLA‐B27, 11 and Edmunds et al found no differences in the features of SpA in patients with five or more attacks of uveitis compared with controls that did not suffer with uveitis.12
We also could not find any features of patients with AAU that could predict recurrence, although it is possible that with a larger study the presence of SpA may prove a significant risk factor. Markers of disease severity have also been suggested as methods of distinguishing those with HLA‐B27 and SpA but there does not appear to be any difference in the rates of hypopyon in HLA‐B27 positive patients with and without SpA2 or rates of visual loss, ocular hypertension or cataract.11
This study also has implications for the etiology of AAU. If there is no demonstrable difference in the course of AAU with demographic variables, HLA status or pattern of arthritis then causes of AAU recurrence must be sought elsewhere. Inconsistent seasonal variation in attacks has been noted.12,13 Stress has been suggested12 but not confirmed14 as a precipitant. This study suggests that AAU patients are relatively homogenous in regard to natural history. The survival estimates and the incidence rates may suggest that the hazard could be increased after one recurrence and the times to the second and third recurrences were considerably shorter compared to time from disease onset to the first recurrence. This implies that as yet unidentified features of the initial attack, or the patient, are a major determinant of whether multiple recurrences will ensue.
Identifying individuals with a higher risk of disease recurrence would provide important information for patients, so allowing them a greater understanding of their condition and acceptance of its management. Whether disease recurrence is linked with a consequent increased risk of complications and a poorer visual outcome is yet to be confirmed. Should this be the case, it would allow the identification of those that will require further investigation at presentation and who, in the future, may benefit from prophylactic treatment.
Abbreviations
AAU - acute anterior uveitis
SpA - spondyloarthropathies
References
- 1.Paivonsalo‐Hietanen T, Vaahtoranta‐Lehtonen H, Tuominen J.et al Uveitis survey at the University Eye Clinic in Turku. Acta Ophthalmol 199472505–512. [DOI] [PubMed] [Google Scholar]
- 2.Power W J, Rodriguez A, Pedroza‐Seres M.et al Outcomes in anterior uveitis associated with the HLA‐B27 haplotype. Ophthalmology 19981051646–1651. [DOI] [PubMed] [Google Scholar]
- 3.Rothova A, van Veenendal W G, Linssen A.et al Clinical features of acute anterior uveitis. Am J Ophthalmol 1987103137–145. [DOI] [PubMed] [Google Scholar]
- 4.Wakefield D, Easter J, Penny R. Clinical features of HLA‐B27 anterior uveitis. Aust J Ophthalmol 198412191–196. [PubMed] [Google Scholar]
- 5.Tay‐Kearney M, Schwam B, Lowder C. Clinical features and associated systemic diseases of HLA‐B27 uveitis. Am J Ophthal 199612147–56. [DOI] [PubMed] [Google Scholar]
- 6.BenEzra D.et al Investigation. In: BenEzra D, Shigeaki O, Secchi GA, Alio LJ, eds. Anterior segment intraocular inflammation guidelines. London, UK: Martin Dunitz, 200029–37.
- 7.Dougados M, van der Linden S, Juhlin R.et al The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum 1991341218–1227. [DOI] [PubMed] [Google Scholar]
- 8. Stata survival analysis and epidemiological tables reference manual release 9; Stata Press, Texas, USA; StataCorp LP, 1985– 2005120–154.
- 9.Feltkamp T E W. HLA‐B27, acute anterior uveitis, and ankylosing spondylitis. Adv Inflamm Res 19859211–216. [Google Scholar]
- 10.Norn M S. Endogenous uveitis. II Recurrence rate. Acta Ophthal 196947366–377. [DOI] [PubMed] [Google Scholar]
- 11.Linssen A, Meenken C. Outcomes of HLA‐B27 positive and HLA‐B27 negative acute anterior uveitis. Am J Ophthalmol 1995120351–361. [DOI] [PubMed] [Google Scholar]
- 12.Edmunds L, Elswood J, Calin A. New light on uveitis in ankylosing spondylitis. J Rheumatol 19911850–52. [PubMed] [Google Scholar]
- 13.Paivoansalo‐Hietanen T, Tuominen J, Matti Saari K. Seasonal variation of endogenous uveitis in south‐western Finland. Acta Ophthalmolo Scand 199876599–602. [DOI] [PubMed] [Google Scholar]
- 14.Secchi A. G, Magni M.D, Tognon M.D. A psychosomatic approach to idiopathic recurrences of anterior uveitis. Am J Ophthalmol 1987104174–178. [DOI] [PubMed] [Google Scholar]