We investigated whether vitreous levels of advanced glycation end products (AGEs) were positively correlated with vascular endothelial growth factor (VEGF) in patients with diabetic retinopathy patients sufficiently treated with retinal photocoagulation. Vitreous AGE and VEGF levels were significantly higher in patients with diabetes than in controls. Positive correlation between AGE and VEGF was found in patients with diabetic retinopathy sufficiently treated with retinal photocoagulation (r = 0.44, p<0.05), but not in those who were insufficiently treated (r = 0.26, p = 0.18). The present observations suggest that AGE may induce VEGF expression in an ischaemia‐independent mechanism. AGE could be one of the important determinants of VEGF in diabetic retinopathy without obvious ischaemic regions.
Background
Vascular endothelial growth factor (VEGF) elicits retinal vascular hyperpermeability, thrombosis and angiogenesis, having a central role in the pathogenesis of diabetic retinopathy.1 Furthermore, vitreous VEGF levels are increased in proliferative diabetic retinopathy, whereas the levels are decreased after treatment with panretinal photocoagulation (PRP).2 These observations suggest that retinal ischaemia and resultant hypoxia could mainly contribute to VEGF induction in diabetic retinopathy.
Advanced glycation end products (AGEs), senescent macroprotein derivatives formed at an accelerated rate under diabetes, also stimulate VEGF expression in cell cultures and animal models.3,4 In addition, vitreous AGE levels are positively correlated with VEGF in patients with diabetic retinopathy, suggesting that AGEs may be a stimulant of VEGF in vivo.5 However, as AGEs predispose the retinal vessels to thrombogenesis,3 whether AGEs could induce VEGF expression in an ischaemia‐independent manner remains unknown. Therefore, in this study, we determined the relationship between vitreous levels of AGEs and VEGF in patients with diabetic retinopathy who were sufficiently treated with PRP for controlling retinal ischaemia.
Patients and methods
The study protocol was approved by our institutional ethics committee, and informed consent was obtained from all patients. Undiluted vitreous samples were collected during vitrectomy from patients with diabetes with a mean (standard deviation (SD)) age of 53 (12.6) years (45 samples from patients with proliferative diabetic retinopathy and 6 from those with diabetic macular oedema). Twenty eight samples from patients without diabetes with a mean (SD) age of 65.3 (6.7) years, having idiopathic macular hole or epiretinal membrane, served as controls. Vitreous levels of AGEs and VEGF were measured as described previously.5 We classified patients with diabetes into two groups: a sufficiently treated group with PRP (S‐PRP; n = 23) and an insufficiently treated group (no or focal retinal coagulation; IS‐RP; n = 28), on the basis of the extent of retinal photocoagulation before vitrectomy as described previously.6 The data were analysed by the Mann–Whitney U test and Spearman's correlation coefficient by rank test.
Results
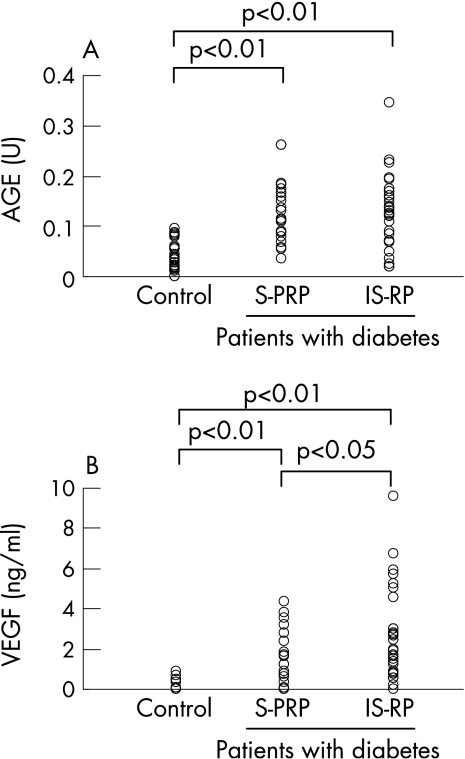
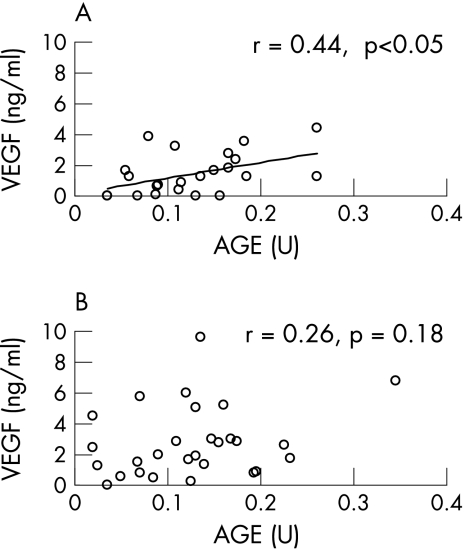
Vitreous levels of AGEs and VEGF were significantly higher in patients with diabetes than in controls (mean (SD) 0.13 (0.07) v 0.04 (0.03) U and 2.15 (2) v 0.12 (0.23) ng/ml, respectively; p<0.01). As fig 1A and B shows, vitreous VEGF levels were higher in IS‐RP than those in S‐PRP (2.75 (2.3) v 1.4 (1.3) ng/ml, respectively; p<0.05), whereas there was no significant difference of vitreous levels of AGEs between IS‐RP and S‐PRP (0.13 (0.07) v 0.13 (0.06) U, respectively). A positive correlation was found between AGEs and VEGF in S‐PRP (r = 0.44, p<0.05), but not in IS‐RP (r = 0.26, p = 0.18; fig 2 A,B).
Figure 1 Vitreous levels of advanced glycation end products (AGEs; A) and vascular endothelial growth factor (VEGF; B) in controls without diabetes and in patients with diabetes, including the sufficiently treated group with panretinal photocoagulation (S‐PRP) and the insufficiently treated group with no or focal photocoagulation (IS‐RP). Data analysed by Mann–Whitney U test.
Figure 2 Correlation between the vitreous levels of advanced glycation end products (AGEs) and vascular endothelial growth factor (VEGF) in the sufficiently treated group with panretinal photocoagulation (S‐PRP; A) and in the insufficiently treated group with no or focal photocoagulation (IS‐RP; B). Spearman's correlation coefficient calculated by rank test.
Comment
Our observations suggest that AGEs may induce VEGF expression in an ischaemia‐independent mechanism. In this study, the positive correlation between vitreous AGEs and VEGF levels disappeared when retinal ischaemia was not sufficiently controlled. Therefore, with the progress of diabetic retinopathy, retinal ischaemia and subsequent hypoxia may become a major determinant of VEGF. Our findings suggest that inhibition of AGE formation could prevent the development of early diabetic retinopathy by suppressing VEGF expression.
Footnotes
Funding: This work was supported in part by Grants of Venture Research and Development Centers from the Ministry of Education, Culture, Sports, Science and Technology, Japan (SY), and the Specific Research Fund of Hokuriku University, Japan (MT).
Competing interests: None declared.
References
- 1.Tolentino M J, Miller J W, Gragoudas E S.et al Intravitreous injections of vascular endothelial growth factor produce retinal ischemia and microangiopathy in an adult primate. Ophthalmology 19961031820–1828. [DOI] [PubMed] [Google Scholar]
- 2.Aiello L P, Avery R L, Arrigg P G.et al Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 19943311480–1487. [DOI] [PubMed] [Google Scholar]
- 3.Yamagishi S, Imaizumi T. Diabetic vascular complications: pathophysiology, biochemical basis and potential therapeutic strategy. Curr Pharm Des 2005112279–2299. [DOI] [PubMed] [Google Scholar]
- 4.Yamagishi S, Amano S, Inagaki Y.et al Advanced glycation end products‐induced apoptosis and overexpression of vascular endothelial growth factor in bovine retinal pericytes. Biochem Biophys Res Commun 200229973–978. [DOI] [PubMed] [Google Scholar]
- 5.Yokoi M, Yamagishi S, Takeuchi M.et al Elevations of AGE and vascular endothelial growth factor with decreased total antioxidant status in the vitreous fluid of diabetic patients with retinopathy. Br J Ophthalmol 200589673–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitamura Y, Takeuchi S, Matsuda A.et al Monocyte chemoattractant protein‐1 in the vitreous of patients with proliferative diabetic retinopathy. Ophthalmologica 2001215415–418. [DOI] [PubMed] [Google Scholar]