Abstract
Aim
To determine the viscoelastic properties of the porcine lens
Methods
Linear viscoelastic shear properties of the stroma of four porcine lenses were measured within 5 hours post‐mortem, using sinusoidal oscillatory shear deformation. The elastic shear modulus, viscous shear modulus, dynamic viscosity, damping ratio, and phase shift of the lenses were quantified by a controlled‐strain, linear simple‐shear rheometer at frequencies of 10–50 Hz.
Results
The mean viscoelastic properties and their standard deviations across the frequencies examined were: the elastic shear modulus, G′ = 6.2±4.0 Pa, the viscous shear modulus, G″ = 19.2±2.5 Pa, the dynamic viscosity, η′ = 0.16±0.1 Pa•sec, the damping ratio ζ = 4.06±1.25, and the phase shift, δ = 76°± 5.6°.
Conclusions
The measured viscoelastic shear properties of the porcine lens reflect a low dynamic viscosity with a high damping ratio. The porcine lens is viscoelastic and is more viscous than elastic. The magnitude of the complex shear modulus of the porcine lens, |G*|, is similar to the shear modulus of the young human lens. Understanding these viscoelastic properties of the natural lens may provide guidance in developing a lens substitute capable of accommodation in the post cataract patient.
Progress in developing an artificial intraocular lens that can change power like the human lens critically depends on a better understanding of the material properties of the crystalline lens. The crystalline lens is viscoelastic.1,2 Its rigidity can be measured by the complex shear modulus,3,4,5 G*, which consists of the real part, the elastic shear modulus, G′, and the imaginary part, the viscous shear modulus, G″. The magnitude of the complex shear modulus, |G*|, is given by:
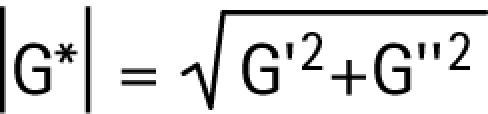 |
The higher the magnitude of the complex shear modulus, the more rigid the lens.
To measure the viscoelastic material properties of the lens, a time varying strain is required. The complex shear modulus, G*, depends on the amplitude and frequency of the deforming strain.3,4,5 By placing the lens between two parallel plates, applying a small‐amplitude sinusoidal oscillatory shear strain, and measuring the resulting shear stress, linear viscoelastic shear properties of the lens can be determined, including; the elastic shear modulus, the viscous shear modulus, the dynamic viscosity, the damping ratio, and the phase shift.2,3,4,5
The dynamic viscosity, η′, is related to the viscous shear modulus by the following equation:
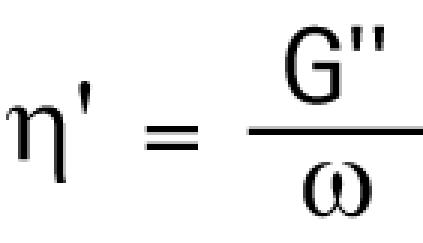 |
where, ω, is the angular frequency of the deforming strain. The higher the dynamic viscosity, the more viscous is the material.
The damping ratio is a measure of the energy loss when the material is subjected to an oscillatory strain. The damping ratio, ζ, is the ratio of the viscous and elastic shear moduli:
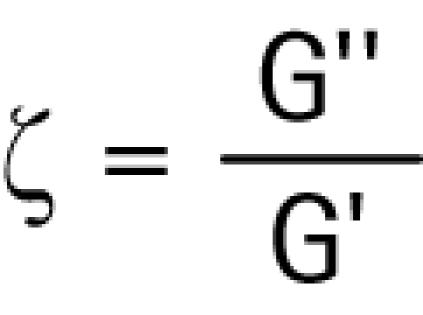 |
When the damping ratio is less than 1.0, equal to 1.0, or greater than 1.0, the material is described, respectively, as underdamped, critically damped, or overdamped.
Another viscoelastic parameter, determined by the viscous and elastic shear moduli, is the phase shift. It is the shift in phase between the oscillatory strain and stress of the lens, and is measured in degrees. It is mathematically expressed as the arctangent of the ratio of the viscous and elastic shear moduli:
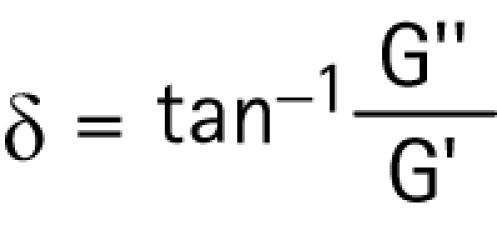 |
When the phase shift falls between zero and ninety degrees, is equal to zero, or is infinite, a material is respectively characterized as being, viscoelastic, purely elastic, or purely viscous.3,4,5
Knowledge of the viscoelastic properties of the crystalline lens may make it possible to duplicate these functional characteristics in a deformable, accommodating lens substitute.
Methods
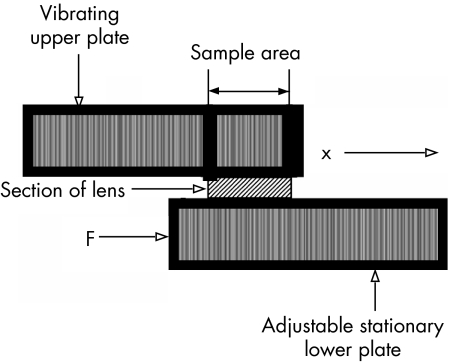
Four eyes from four, 5‐month old pigs were obtained from an abattoir immediately following death and transported on ice in less than 30 minutes to our laboratory. The clear intact lens was carefully removed from the eye. The lens capsule was dissected from the stroma of each lens. A one‐millimeter thick mid‐sagittal section was excised from two of the lenses. A one‐millimeter mid‐transverse section was excised from the other two lenses. Each lens section was placed between two parallel acrylic plates of a calibrated validated controlled‐strain, linear simple‐shear rheometer6 (EnduraTec ELF 3200, Bose Corporation, Minnetonka, MN). The rheometer consisted of a linear motor or actuator capable of prescribing a precise, small‐amplitude sinusoidal shear displacement to the excised block of fresh lens stroma via the upper plate (fig 1). The sinusoidal shear force resulting from the viscoelastic response of the lens stroma was detected by a piezoelectric quartz force transducer (PCB 209C12, PCB Piezotronics, Depew, NY, USA) attached to the lower plate. Additionally, the lower plate was attached to a micrometer. Before each test, the micrometer was adjusted until both the upper plate and the lower plate surfaces were in contact with the entire surface of the lens section. The distance between these plates was 1 mm. The plates were enclosed in a chamber to control temperature at 37°±0.1°C and humidity at 80%.
Figure 1 Schematic diagram of the linear, simple‐shear rheometer. The section of porcine lens was subjected to a linear sinusoidal shear strain as applied by the controlled displacement of the upper plate (x), and the shear force resulting from the viscoelastic response of the sample (F) was detected by a piezoelectric force transducer attached to the lower plate.
Using a camera that was centered above the lens section and rigidly mounted to the rheometer, a digital photograph was taken of the area of the lens section in contact with the upper plate, before and after completing the testing, to verify stability of the contact surface during the measurement, and to allow for the calculation of shear stress. To ensure stress‐strain linearity and negligible inertia of the sample, the displacement of the upper plate was set at 1% of the distance between the plates (i.e., 1% shear strain).5
The principle of controlled strain rheometry3,4,5 was followed, with sinusoidal oscillatory shear deformation applied to the lens stroma in the following steps: 10 Hz, 15 Hz, 20 Hz, 25 Hz, and 50 Hz. The measurements of each lens section took approximately 20 minutes. All measurements commenced within 5 hours of the pigs' death and within 5 minutes of removal of the lenses from the eyes.
For each frequency, the changes in phase and amplitude of 20 cycles of oscillations were measured. Then, the means and standard deviations of G′, G″, η′, and ζ at each frequency for all the lenses were calculated according to the theory of linear viscoelasticity,6 using Matlab (Version 7.0, The Mathworks, Inc., Natick, MA, USA) and plotted on a log‐log scale.
Results and discussion
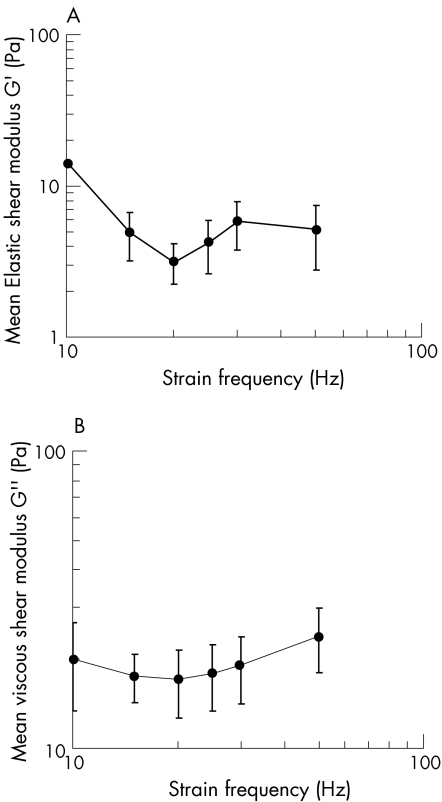
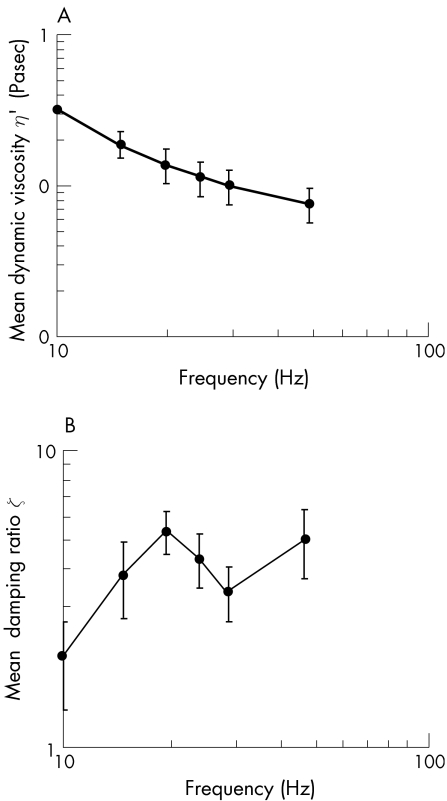
The mean changes in G′, G″, η′, and ζ versus frequency are given in figs 2 and 3. The mean G′, G″, η′, and ζ for all frequencies were 6.2±4.0 Pa, 19.2±2.5 Pa, 0.16±0.1 Pa•sec and 4.06±1.25, respectively. The phase shift, δ, = tan‐1(ζ) was 76°±5.6°. There was no significant difference in response of the mid‐sagittal and mid‐transverse sections.
Figure 2 A. Mean elastic shear modulus (± standard deviations) of the porcine lens stroma as a function of frequency (n = 4). B. Mean viscous shear modulus (± standard deviations) of the porcine lens stroma as a function of frequency (n = 4).
Figure 3 A. Mean dynamic viscosity (± standard deviations) of the porcine lens stroma as a function of frequency (n = 4). B. Mean damping ratio (± standard deviations) of the porcine lens stroma as a function of frequency (n = 4).
Since the mean phase shift for the porcine lens was found to equal 76°, by definition, the lens stroma is viscoelastic. The mean elastic shear modulus of the porcine lens is less than its mean viscous shear modulus. Therefore, the porcine lens stroma can be described as more viscous than elastic. The mean dynamic viscosity of the porcine lens is low and, as is common with soft biological tissues,5 decreases linearly and monotonically with frequency on a log‐log scale. Since the damping ratio was greater than 1.0 for all frequencies, the porcine lens is over‐damped.
The magnitude of the complex shear modulus of the porcine lens is 20.2 Pa. This shear modulus is about 40% of the magnitude of the mean shear modulus of the young human lens, which is 50 Pa.7,8
Since the speed of ultrasound,9,10 the biochemistry11,12 and the structure13,14 of porcine and human lenses are similar, it is likely that the human lens, like the porcine lens, also has a low dynamic viscosity and is over‐damped. Over‐damping of the human lens can be demonstrated, in vivo, during human accommodation. It is reflected in the ability of the eye to rapidly and consistently track a step stimulus that is within the linear portion of the subject's accommodative function.15 Overdamping of the lens is also demonstrated in the linear portion of accommodation by the rapid decline that occurs in the magnitude of the power spectrum of the microfluctuations.15
In summary, the porcine lens is viscoelastic, readily deformed, and overdamped. It has a shear modulus similar in magnitude to that of the young human crystalline lens. Understanding these viscoelastic properties of the natural lens may provide guidance in the development of a deformable lens substitute, capable of accommodation in the post cataract patient.
Acknowledgments
Funding: This study is partially supported by a grant from the National Institutes of Health, NIDCD Grant no. R01 DC006101.
Footnotes
Competing interest: RAS has a financial interest in the surgical reversal of presbyopia
References
- 1.Kikkawa Y, Sato T. Elastic properties of the lens. Exp Eye Res 19632210–215. [DOI] [PubMed] [Google Scholar]
- 2.Fisher R F. The elastic constants of the human lens, J Physiol (London) 1971212147–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fung Y C. Biomechanics. mechanical properties of living tissues. 2nd ed. New York: Springer, 199323–65.
- 4.Lutz R J, Litt M, Chakrin L W. Physical‐chemical factors in mucus biology. In: Gabelnick HL, Litt M, eds. Rheology of biological systems. Springfield, IL: Charles C, Thomas Publishers 1973119–157.
- 5.Chan R W, Titze I R. Viscoelastic shear properties of human vocal fold mucosa: Measurement methodology and empirical results. J Acoust Soc Am 19991062008–2021. [DOI] [PubMed] [Google Scholar]
- 6.Chan R W, Rodriguez M, Lee B. Validation of a controlled‐strain simple shear rheometer for vocal fold tissue characterization. The Society of Rheology, 76th Annual Meeting. Feb 17, 2005;Paper EM13. Abstract
- 7.Heys K R, Cram S L, Truscott R J. Massive increase in the stiffness of the human lens nucleus with age: the basis for presbyopia? Mol Vis 200410956–963. [PubMed] [Google Scholar]
- 8.Schachar R A, Abolmaali A, Kamangar F. Comment on the publication “Three‐dimensional ultrasound, biomicroscopy environmental and conventional scanning electron microscopy investigations of the human zonula ciliaris for numerical modelling of accommodation” by O. Stachs et al. Graefes Arch Clin Exp Ophthalmol 20062441062–1063. [DOI] [PubMed] [Google Scholar]
- 9.Gorig C, Varghese T, Stiles T.et al Evaluation of acoustic wave propagation velocities in the ocular lens and vitreous tissues of porcines, dogs, and rabbits. Am J Vet Res 200667288–295. [DOI] [PubMed] [Google Scholar]
- 10.Beers A P, Van der Heijde G L. Presbyopia and velocity of sound in the lens. Optom Vis Sci 199471250–253. [DOI] [PubMed] [Google Scholar]
- 11.Liao J H, Hung C C, Lee J S.et al Characterization, cloning, and expression of porcine alpha B crystallin. Biochem Biophys Res Commun 1998244131–137. [DOI] [PubMed] [Google Scholar]
- 12.Trifonova N, Kalaydjiev S, Stamenova M.et al Porcine eye lens crystallins: antigenic similarity with human crystallins and tool for the detection of anti‐crystallin antibodies. Graefes Arch Clin Exp Ophthalmol 2002240777–781. [DOI] [PubMed] [Google Scholar]
- 13.Kuszak J R, Zoltoski R K, Sivertson C. Fibre cell organization in crystalline lenses. Exp Eye Res 200478673–687. [DOI] [PubMed] [Google Scholar]
- 14.Prince J H, Diesem C D, Eglitis I.et al Anatomy and Histology of the Eye and Orbit in Domestic Animals. Springfield, Illinois: Charles C, Thomas, Publisher, 1960210–233.
- 15.Mordi J A, Ciuffreda K J. Dynamic aspects of accommodation: age and presbyopia. Vision Res 200444591–601. [DOI] [PubMed] [Google Scholar]