Abstract
Objective
To evaluate the outcome of autologous retinal pigment epithelium (RPE)–choroid sheet transplantation after removal of a subfoveal choroidal neovascularisation (CNV) in patients with age related macular degeneration (AMD).
Methods
RPE–choroid sheet transplantation was performed in 10 consecutive patients with exudative AMD (n = 9) or geographic atrophy (n = 1). After CNV extraction, an autologous RPE–choroid patch was translocated from the midperiphery under the macula. Follow‐up was between 6 and 12 months. Visual acuity testing and microperimetry (Nidek‐MP1) as well as autofluorescence, fluorescein and indocyanine green (ICG) angiography were performed and the data were analysed retrospectively.
Results
Visual acuity (logarithm of minimum angel of resolution) before operation ranged from 0.7 to 1.8 (mean 1.37) and after operation from 0.4 to 1.6 (mean 1.24). Visual acuity after operation improved in seven patients (by a mean of 0.26), remained stable in one patient and decreased in two patients. Microperimetry showed light sensitivity and fixation on the sheet in five cases. ICG angiography demonstrated perfusion through the RPE–choroid graft in nine patients. Postoperative complications included retinal detachment (n = 1) and epiretinal membrane formation (n = 2). The patient with geographic atrophy developed a CNV after surgery.
Conclusions
Autologous RPE–choroid sheet transplantation is feasible and a comparatively safe procedure. Microperimetry showed fixation and light perception over the graft with a moderate increase in mean visual acuity.
The current treatment of exudative age related macular degeneration (AMD) is based on thermal laser surgery, photodynamic therapy and quite recently intravitreal anti‐vascular endothelial growth factor therapy.1,2,3,4,5,6 Using these techniques the underlying course of the disease can be modulated but not erased. Surgical treatment is one method of removing choroidal neovascularisation (CNV) completely. However, removal of the CNV irreversible damages the functionality of the retinal pigment epithelium (RPE), Bruch's membrane and choriocapillaris, compromising the clinical success.7,8,9,10
The clinical results of macula rotation surgery showed that extra‐foveal RPE can support foveal photoreceptor function.11,12 However, the rather high rate of complications (PVR formation, diplopia, macular oedema) is a major limitation of this complex procedure. Another option is transplantation of healthy RPE to the subfoveal space. Subretinal transplantation of human RPE allografts in patients with AMD has shown no substantial visual benefit because of delayed immunoreaction resulting in subretinal fibrosis.13,14 Subfoveal injection of autologous RPE cells or autologous iris pigment epithelial cells also did not significantly improve visual function, and the adherence and polarisation of the injected cells on the damaged Bruch's membrane remains uncertain.15,16
In 2002, Stanga et al first described subfoveal translocation of an autologous RPE–choroid sheet that was cut out from the edge of the RPE defect after CNV extraction.17 They demonstrated fixation and light perception on the sheet area in seven of nine patients but visual acuity did not improve. Van Meurs and Van Den Biesen first described subfoveal transplantation of a RPE–choroid patch that was cut out from the superior midperiphery in six patients.18 They demonstrated fixation on the graft and improvement in visual acuity by two lines in three of these patients.
Based on these encouraging results, we present a series of 10 patients who underwent CNV extraction and subfoveal translocation of an autologous midperipheral RPE–choroid sheet. In this retrospective analysis, we report our morphological findings and functional results.
Patients and methods
In this interventional case series, 10 patients underwent autologous RPE–choroid transplantation between September 2004 and August 2005. Median age was 77 years (range 52–84) and median follow‐up time was 10.5 months (range 6–12). The data were analysed retrospectively.
The indication for surgery was severe loss of central vision due to massive subretinal haemorrhage in five eyes, a large occult neovascular membrane in three eyes, and pigment epithelium detachment and non‐exudative geographic atrophy in one eye each.
All patients underwent a standard three port vitrectomy with a clean posterior hyaloidal detachment. Removal of the CNV was performed through a retinotomy adjacent to the temporal upper vein. The RPE–choroid patch was prepared as follows. In the superior midperiphery, the retina was coagulated with 500 ms of a green Nd:YAG laser with several rows of laser burns in a circle, using power settings producing a chalk white lesion. The retina was then locally detached within the coagulated circle using the DORC Teflon cannula and removed with a cutter. The neurotomy knife (Synergetics Inc., USA) was used to make a first incision in the RPE and the choroid. Special developed scissors (Geuder, Heidelberg, Germany) were used to cut out a patch consisting of RPE and superficial choroid. The patch was reinserted through the retinotomy using subretinal forceps to place the patch beneath the detached macular retina as centrally as possible. Heavy liquid was used to flatten the retina and was replaced with silicon oil for endotamponade.
Postoperative visits were scheduled at 1.5, 3, 6, 9 and 12 months. Silicon oil removal was performed after 6 months in seven patients. Each postoperative visit included best corrected visual acuity testing by refraction (logarithm of minimum angel of resolution (logMAR): counting fingers was converted to logMAR 1.6, hand motion to logMAR 1.8) and full ophthalmoscopic evaluation.
Retinal autofluorescence, fluorescein and indocyanine green (ICG) angiography were recorded with a scanning laser ophthalmoscope (HRA2; Heidelberg Engineering, Heidelberg, Germany).
Computer assisted fundus correlated microperimetry with the MP1 microperimeter (Nidek Technologies, Padova, Italy) was used to prove fixation and retinal sensitivity over the RPE–choroid patch (manual or semiautomatic static threshold 4–2 strategy).
In all patients, the type of surgery and alternative options were explained in detail. Informed consent was obtained before surgery in all cases. Ethics committee approval for this interventional case series was not obtained.
Results
Table 1 summarises the most relevant clinical and patient data. In table 1, preoperative duration of visual loss describes subjective and objective recent disease progression due to retinal haemorrhage or enlargement of the occult CNV, or pigment epithelium detachment or geographic atrophy, respectively. We did not perform silicon oil removal in patient 1 because of a proliferative vitreoretinopathy (PVR) retinal detachment and in patient 4 because of severe epiretinal membrane formation requiring further endotamponade. In patient 10, silicon oil removal was planned contemporarily. Patients 3, 6 and 10 had been pseudophakic prior to surgery; all other patients underwent combined phacoemulsification, intraocular lens implantation and RPE–choroid sheet transplantation.
Table 1 Patient details.
| Patient No | Sex | Age (y) | Indication | Duration of visual loss | Preoperative visual acuity (logMAR) | Postoperative visual acuity (logMAR) | Fixation on graft by microperimetry | Complications | Silicone oil removal | Follow‐ up (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 74 | Subretinal haemorrhage | 2 wk | 0.9 | 1.4 | No | Retinal detachment | No | 12 |
| 2 | F | 83 | Subretinal haemorrhage | 2 wk | 0.7 | 0.4 | Yes | None | Yes | 12 |
| 3 | F | 80 | Subretinal haemorrhage | 4 wk | 1.8 | 1.3 | No | None | Yes | 12 |
| 4 | M | 84 | Subretinal haemorrhage | 1 wk | 1.8 | 1.6 | No | Severe epiretinal membrane formation | No | 12 |
| 5 | F | 76 | Occult CNV | 3 mo | 1.4 | 1.3 | Yes | None | Yes | 12 |
| 6 | F | 73 | PED | 3 mo | 1.3 | 1.0 | Yes | Subtle epiretinal gliosis | Yes | 9 |
| 7 | F | 52 | Occult CNV | 2 mo | 1.6 | 1.6 | no | None | Yes | 6 |
| 8 | M | 81 | Occult CNV | 3 mo | 1.3 | 0.8 | Yes | None | Yes | 6 |
| 9 | F | 78 | Subretinal haemorrhage | 1 wk | 1.6 | 1.4 | Yes | None | Yes | 6 |
| 10 | F | 68 | Geographic atrophy | 6 mo | 1.3 | 1.6 | No | CNV | No | 6 |
CNV, choroidal neovascularisation; F, female; logMAR, logarithm of minimum angel of resolution; M, male.
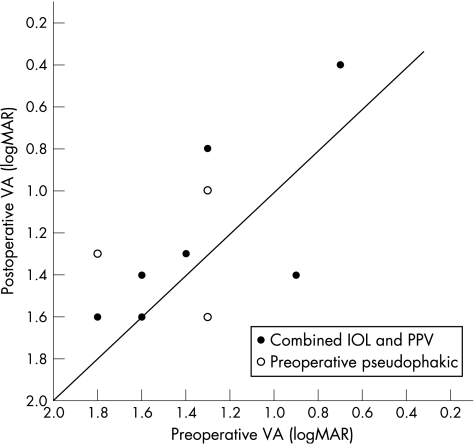
Visual acuity (logMAR) ranged from 0.7 to 1.8 (mean 1.37) before operation and from 0.4 to 1.6 (mean 1.24) after operation. Figure 1 shows visual acuity before and after operation for each individual patient. Seven patients had mild or moderate senile cataract before operation; they underwent combined phacoemulsification, intraocular lens implantation and RPE–choroid sheet transplantation. Three patients were already pseudophakic before surgery. Visual acuity after operation improved in seven patients but the improvement was only moderate (by mean 0.26 logMAR) and in five of these seven patients visual acuity remained 1.0 (logMAR) or worse. Visual acuity decreased in two patients due to PVR retinal detachment and postoperative CNV, respectively.
Figure 1 Preoperative compared with postoperative visual acuity (VA) at the end of the follow‐up period for each patient. Seven patients underwent combined phacoemulsification, intraocular lens (IOL) implantation and pars plana vitrectomy (PPV). Three patients were already pseudophakic before surgery.
Fundus correlated microperimetry showed perception of light stimuli (Goldmann III, 200 ms) and predominantly stable fixation on the RPE patch in five of 10 patients. In cases where retinal sensitivity and fixation on the graft was demonstrated within 3 months after surgery, it was maintained until the end of the follow‐up period. In contrast, if there was no microperimetric function detectable within 3 months, it was not achieved during the follow‐up period.
Angiography was used to assess vascularisation of the graft. Fluorescein angiography was not helpful in analysing the capillary network of the choroid whereas ICG clearly demonstrated a vascular network in or beneath the transplanted RPE patch. Within 1 week after surgery, the choroidal vessel architecture was visible in only one of eight patients. After 1 month it was visible in six of seven patients, after 3 months in all eight patients, after 6 months it was visible in six of seven patients and after 12 months in all three patients examined (table 2).
Table 2 Choroidal perfusion of the retinal pigment epithelium–choroid patch during the follow‐up period, as assessed by indocyanine green angiography.
| Patient No | Choroidal perfusion during follow‐up | ||||
|---|---|---|---|---|---|
| <1 week | 1 month | 3 months | 6 months | 12 months | |
| 1 | – | + | + | + | nt |
| 2 | nt | nt | + | + | nt |
| 3 | Blood | Blood | + | + | + |
| 4 | – | + | + | + | + |
| 5 | – | + | + | + | + |
| 6 | – | – | + | nt | |
| 7 | – | + | nt | nt | |
| 8 | – | + | + | nt | |
| 9 | + | + | + | + | |
| 10 | – | nt | nt | – | |
+ perfusion visible; –perfusion not visible; Blood, blocked fluorescence because of blood; nt, not tested.
Autofluorescence was absent in the area devoid of native RPE after CNV extraction. During the follow‐up period, the translocated RPE–choroid patch fluoresced with intensity similar to the native RPE in nine patients. Patient 10 (geographic atrophy) showed a reduced autofluorescence of the graft after 3 months and angiography revealed the development of a CNV at this time. Figure 2 shows the angiographic and microperimetric findings of a successfully transplanted RPE–choroid sheet.
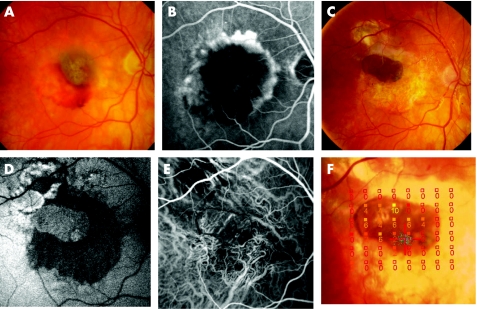
Figure 2 (A–F) Patient 8 presented an occult choroidal neovascularisation with subretinal haemorrhage before operation. Fundus photography (A) and corresponding fluorescein angiography (B); visual acuity = 1.3 logarithm of minimum angel of resolution (logMAR). Six months after surgery, visual acuity improved to 0.16 logMAR. The pigmented retinal pigment epithelium (RPE)–choroid patch is well visible (C). Autofluorescence of the graft was well preserved (D) and indocyanine green angiography shows choroidal vascularisation in or beneath the RPE–choroid sheet (E). Microperimetry showed fixation (blue spots) and retinal sensitivity over the patch area (filled squares, dB light increment sensitivity) (F).
In all patients it was possible to translocate a full thickness graft of RPE and choroid to the subfoveal position. Intraoperative complications did not occur. Bleeding, in particular, from the choroid was not observed. Curling of the RPE–choroid patch was reduced by preparing a round patch. The most difficult part was found to be placement of the patch underneath the subfoveal retina.
One patient experienced retinal detachment under silicone oil 5 weeks after operation, requiring further surgery. In one patient distinct paracentral epiretinal membrane formation was observed and another patient developed a subtle macular pucker that was peeled during vitrectomy for silicon oil removal. Postoperative recurrence of a CNV was not observed. However, patient 10 presented a non‐exudative geographic atrophy before operation and developed a CNV 3 months after surgery (fig 3).
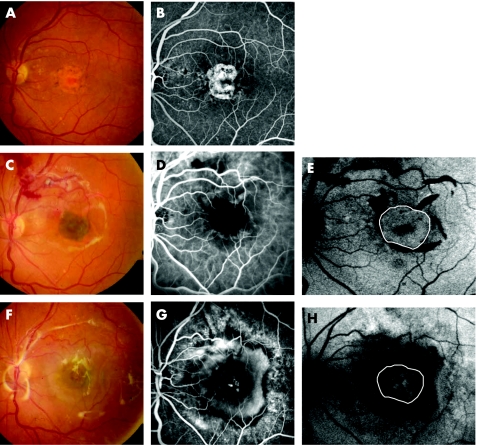
Figure 3 (A–H) Patient 10 presented a geographic atrophy before operation. Fundus photography (A) and corresponding fluorescein angiography (B); visual acuity = 1.3 logarithm of minimum angel of resolution (logMAR). Five days after surgery the retinal pigment epithelium–choroid patch is visible in the fovea (C), vascularisation in the patch area is not visible on indocyanine green angiography (D) but the autofluorescence of the graft is still preserved (E) (white circle). Three months after surgery, a large choroidal neovascularisation developed. Fundus photography (F) and fluorescein angiography (G). Autofluorescence is markedly reduced in the area of the graft (white circle) (H) and visual acuity decreased to 1.4 logMAR.
Discussion
In this study, translocation of the RPE–choroid sheet to the subfoveal space was feasible in all 10 patients. We observed no intraoperative complications, particularly no choroidal bleeding. As we did heavy laser coagulation surrounding the midperipheral explantation site, elevating the infusion bottle to avoid choroidal bleeding, as recommended by Stanga et al, was not necessary.17 Because of concerns of loosing the RPE–choroid patch, we used forceps to hold the patch during preparation and subfoveal translocation and not an aspiration–reflux spatula, as described by Van Meurs and Van Den Biesen.18
Postoperative complications included a PVR retinal detachment that required further surgical intervention in one patient. A distinct paracentral epiretinal membrane formation and a subtle macular pucker was observed in one patient each. This corresponds with findings of Stanga et al and Van Meurs and Van Den Biesen. After RPE–choroid sheet transplantation they described a PVR retinal detachment in one of nine patients and a fine epiretinal membrane formation in one of six patients, respectively.17,18
Visual acuity improved in seven cases but the improvement was only moderate (mean 0.26 logMAR). This supports the findings of Van Meurs and Van Den Biesen. They reported a two line increase in visual acuity after autologous midperipheral RPE–choroid sheet transplantation in three of six patients.18 One year earlier, Stanga et al described autologous RPE–choroid sheet transplantation in a series of nine patients.17 They could only stabilise but not improve visual function. One reason for the disappointing visual results could be photoreceptor damage or disarrangement during the CNV extraction and surgical manipulation in the submacular space. Another cause could be the longstanding visual loss before surgery with an already impaired function of the photoreceptors and the outer neurosensory retina preoperatively. In addition, in the series of Stanga et al, the RPE–choroid patch was cut out directly at the edge of the macular RPE defect and not in the midperipheral fundus. The authors supposed that the RPE adjacent to the extracted CNV might have already been affected by AMD or sustained additional trauma during surgical manipulation. Apart from this possible disadvantage, we consider it important to minimise surgical trauma to the extrafoveal retina because it may become the preferred area of fixation once the foveal fixation is lost.
ICG angiography was analysed to assess the vascularisation of the RPE–choroid patch. ICG clearly demonstrated a vascular network in or under the transplanted patch in nine of 10 patients. Presumably, some perfusion of the patch can occur either by reconnection of the vasculature of the graft to the remaining subfoveal choroidal vessels or by new vessels growing into the graft. However, one must have in mind that on the basis of the angiographic findings, it is difficult to determine whether the visible vessels in the patch area actually represent newly ingrown or reconnected vessels. It might be helpful to analyse the pattern of the choroidal vessels as the translocated equatorial choroid has more parallel vasculature compared with the foveal choroid which is more like a cobweb.19,20 It seems unlikely that the visible vessel network in the patch area belongs solely to the underlying deep choroidal vasculature shining through the sheet without any perfusion of the patch itself. In addition, a scanning laser ophthalmoscope revealed almost normal fundus autofluorescence of the RPE–choroid patch during the follow‐up in nine of the 10 patients. This points towards an ongoing interaction between photoreceptors and the retinal pigment epithelium.21 The autofluorescence of the RPE–choroid patch might last for some time even without photoreceptor function because the graft contains lipofuchsine derived from the equatorial photoreceptors. However, fundus‐correlated microperimetry showed fixation and retinal sensitivity over the patch for up to one year after surgery, indicating viable photoreceptors in this area. These findings indicate a viable graft with successful revascularisation.
In our study, one patient presented with a non‐exudative geographic atrophy before operation. Interestingly, this patient showed decreasing autofluorescence of the RPE–choroid graft and a large CNV 3 months after surgery. It is noteworthy that in this case the subfoveal choroidea was gently scratched with a subretinal spatula in order to facilitate the ingrowth of choroidal vessels after longstanding RPE and choriocapillaris atrophy (Kirchhoff B, 2004, personal communication). This manipulation could have encouraged the development of a CNV. In all other cases recurrence of the CNV was not detected after surgery and it seems unlikely that the patch itself would stimulate the growth of a CNV.
Recently, MacLaren et al published the first long term results of RPE–choroid sheet transplantation in four patients.22 Using high resolution optical coherence tomography, they confirmed the presence of an optically dense subretinal mass consistent with survival of the RPE–choroid graft in all patients. However, the overlying retina was abnormal. The optically clear outer retinal zone, generally accepted to correspond to the outer nuclear and photoreceptor layer, was absent in the retina overlying the graft in all cases. The autofluorescence over the patch had also disappeared. These morphological changes were reflected in loss of foveal fixation and further decline of visual acuity. The authors stated that it is not clear whether or not the primary cause of failure can be attributed to the RPE–choroid graft or the photoreceptors. Delayed apoptosis of photoreceptors, set in motion by the surgical trauma or the AMD, is conceivable as in several animal models photoreceptors have been shown to be highly sensitive to apoptosis after disruption of the physiological outer segment–RPE interface.23,24 If the RPE–choroid patch is supposed to be the primary cause of late failure, one should take into consideration that in the above mentioned study the RPE patch was harvested from the macular region adjacent to the extracted CNV. Therefore, it may have already been affected by AMD leading to late failure of the graft.17
In conclusion, autologous midperipheral RPE–choroid patch transplantation is feasible and a comparatively save procedure. Vascularisation of the graft is discernable in almost all cases but cannot be definitely proved using ICG angiography. Fundus correlated microperimetry showed fixation and light perception over the graft, indicating viable RPE cells and functioning overlying photoreceptors in patients with a follow‐up of 1 year. Careful long term follow‐up is needed to determine whether this technique can be a valuable approach in the future management of AMD.
Abbreviations
AMD - age related macular degeneration
CNV - choroidal neovascularisation
ICG - indocyanine green
logMAR - logarithm of minimum angel of resolution
PVR - proliferative vitreoretinopathy
RPE - retinal pigment epithelium
Footnotes
Competing interests: None.
References
- 1.Macular Photocoagulation Study Group Argon laser photocoagulation for neovascular maculopathy. Three‐year results from randomized clinical trials. Arch Ophthalmol 1986104694–701. [PubMed] [Google Scholar]
- 2.Macular Photocoagulation Study Group Argon laser photocoagulation for neovascular maculopathy. Five‐year results from randomized clinical trials. Arch Ophthalmol 19911091109–1114. [PubMed] [Google Scholar]
- 3. Photodynamic therapy of subfoveal choroidal neovascularization in age‐related macular degeneration with verteporfin: one‐year results of 2 randomized clinical trials—TAP report. Treatment of age‐related macular degeneration with photodynamic therapy (TAP) Study Group. Arch Ophthalmol 19991171329–1345. [PubMed] [Google Scholar]
- 4. Verteporfin therapy of subfoveal choroidal neovascularization in age‐related macular degeneration: two‐year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization—verteporfin in photodynamic therapy report 2. Am J Ophthalmol 2001131541–560. [DOI] [PubMed] [Google Scholar]
- 5.Chapman J A, Beckey C. Pegaptanib: a novel approach to ocular neovascularization. Ann Pharmacother 2006401322–1326. [DOI] [PubMed] [Google Scholar]
- 6.Heier J S, Antoszyk A N, Pavan P R.et al Ranibizumab for treatment of neovascular age‐related macular degeneration: a phase I/II multicenter, controlled, multidose study. Ophthalmology 2006113642–644. [DOI] [PubMed] [Google Scholar]
- 7.Eckardt C. Surgical removal of submacular neovascularization membranes. Ophthalmologe 199693688–693. [DOI] [PubMed] [Google Scholar]
- 8.Gass J D. Biomicroscopic and histopathologic considerations regarding the feasibility of surgical excision of subfoveal neovascular membranes. Am J Ophthalmol 1994118285–298. [PubMed] [Google Scholar]
- 9.Hawkins B S, Bressler N M, Miskala P H.et al Surgery for subfoveal choroidal neovascularization in age‐related macular degeneration: ophthalmic findings: SST report no. 11. Ophthalmology 20041111967–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas M A, Dickinson J D, Melberg N S.et al Visual results after surgical removal of subfoveal choroidal neovascular membranes. Ophthalmology 19941011384–1396. [DOI] [PubMed] [Google Scholar]
- 11.Aisenbrey S, Lafaut B A, Szurman P.et al Macular translocation with 360 degrees retinotomy for exudative age‐related macular degeneration. Arch Ophthalmol 2002120451–459. [DOI] [PubMed] [Google Scholar]
- 12.Eckardt C, Eckardt U, Conrad H G. Macular rotation with and without counter‐rotation of the globe in patients with age‐related macular degeneration. Graefes Arch Clin Exp Ophthalmol 1999237313–325. [DOI] [PubMed] [Google Scholar]
- 13.Algvere P V, Berglin L, Gouras P.et al Transplantation of fetal retinal pigment epithelium in age‐related macular degeneration with subfoveal neovascularization. Graefes Arch Clin Exp Ophthalmol 1994232707–716. [DOI] [PubMed] [Google Scholar]
- 14.Algvere P V, Gouras P, Dafgard K E. Long‐term outcome of RPE allografts in non‐immunosuppressed patients with AMD. Eur J Ophthalmol 19999217–230. [DOI] [PubMed] [Google Scholar]
- 15.Lappas A, Foerster A M, Weinberger A W.et al Translocation of iris pigment epithelium in patients with exudative age‐related macular degeneration: long‐term results. Graefes Arch Clin Exp Ophthalmol 2004242638–647. [DOI] [PubMed] [Google Scholar]
- 16.Van Meurs J C, ter Averst E, Hofland L J.et al Autologous peripheral retinal pigment epithelium translocation in patients with subfoveal neovascular membranes. Br J Ophthalmol 200488110–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stanga P E, Kychenthal A, Fitzke F W.et al Retinal pigment epithelium translocation after choroidal neovascular membrane removal in age‐related macular degeneration. Ophthalmology 20021091492–1498. [DOI] [PubMed] [Google Scholar]
- 18.Van Meurs J C, Van Den Biesen P R. Autologous retinal pigment epithelium and choroid translocation in patients with exudative age‐related macular degeneration: short‐term follow‐up. Am J Ophthalmol 2003136688–695. [DOI] [PubMed] [Google Scholar]
- 19.Hayreh S S. Vascular pattern of the choriocapillaris. Exp Eye Res 197419101–104. [DOI] [PubMed] [Google Scholar]
- 20.Hayreh S S. Submacular choroidal vascular pattern. Experimental fluorescein fundus angiographic studies. Albrecht Von Graefes Arch Klin Exp Ophthalmol 1974192181–196. [DOI] [PubMed] [Google Scholar]
- 21.von Ruckmann A, Fitzke F W, Bird A C. Distribution of pigment epithelium autofluorescence in retinal disease state recorded in vivo and its change over time. Graefes Arch Clin Exp Ophthalmol 19992371–9. [DOI] [PubMed] [Google Scholar]
- 22.MacLaren R E, Bird A C, Sathia P J.et al Long‐term results of submacular surgery combined with macular translocation of the retinal pigment epithelium in neovascular age‐related macular degeneration. Ophthalmology 20051122081–2087. [DOI] [PubMed] [Google Scholar]
- 23.Hisatomi T, Sakamoto T, Goto Y.et al Critical role of photoreceptor apoptosis in functional damage after retinal detachment. Curr Eye Res 200224161–172. [DOI] [PubMed] [Google Scholar]
- 24.Yang L, Bula D, Arroyo J G.et al Preventing retinal detachment‐associated photoreceptor cell loss in Bax‐deficient mice. Invest Ophthalmol Vis Sci 200445648–654. [DOI] [PubMed] [Google Scholar]