Abstract
Aim
To examine the association of reported visual hallucinations and measured visual parameters in adult patients referred for low vision rehabilitation.
Methods
All patients (N = 225) referred to a low vision rehabilitation clinic for a calendar year were asked a standardised question about symptoms of formed visual hallucinations. Best corrected visual acuity and contrast sensitivity using the Pelli‐Robson chart were measured. We conducted multiple logistic regression analysis of the association between visual hallucinations and visual parameters.
Results
Of the total cohort, 78 (35%) reported visual hallucinations. Visual acuity and contrast sensitivity were considered in four quartiles. In multiple logistic regression controlling for contrast sensitivity, age, gender, report of depression and independence, measured acuity in each of the poorer three categories (compared to the best) was not associated with reported hallucinations. Contrast sensitivity in the three poorer quartiles (compared to the best) was strongly associated with the report of hallucinations (OR 4.1, CI 1.1, 15.9; OR 10.5, CI 2.6, 42.1; OR 28.1, CI 5.6, 140.9) after controlling for acuity, age, sex, depression and independence.
Conclusions
Lowest contrast sensitivity was the strongest predictor of reported hallucinations after adjusting for visual acuity.
Keywords: Charles Bonnet hallucinations, contrast sensitivity
Patients with visual impairment report visual hallucinations, such as seeing miniature people or complex scenery. Such visual hallucinations have been called Charles Bonnet hallucinations after the philosopher who first described hallucinations experienced by his visually impaired grandfather in 1769.1 The most common explanation of such hallucinations is that a lack of true visual input facilitates a cortical release phenomena of visual images.2
In a previous report we described our prospective study of a low vision rehabilitation population and the impact of vision rehabilitation on functional capacity and morbidity.3 In this paper we examine the relationship between visual hallucinations, visual acuity and measured contrast sensitivity.
Methods
After institutional ethics approval, 225 patients who were referred for the first time to our vision rehabilitation service agreed to participate and to sign informed consent. Patients had previously had an examination by an ophthalmologist and had received, or were receiving, available treatment. Although all patients had functional difficulty related to their vision loss, which lead to them seeking rehabilitation, there was variation in the patients' diagnoses, duration and degree of visual loss.
Each patient was told, “Some patients with partial vision who come to the Clinic tell us that they see things that they know are not there. They may see coloured shapes or organised patterns or they may even see vivid images of people, animals or flowers.” They were then asked, “ Have you ever experienced this?” Only reports of formed images were considered hallucinations and reports of photopsias or brief flashes of light were not categorised as visual hallucinations. Best corrected visual acuity was measured at 6 meters or at 1 meter if the acuity was less than 6/24. Contrast sensitivity was measured with the Pelli‐Robson chart at 1 metre. The Pelli‐Robson contrast sensitivity chart presents 16 triplets of letters in decreasing shades of grey. The Pelli‐Robson scoring sheet tabulates measured contrast threshold in logarithmic increments of 0.15, from 0.00 representing that fewer than five letters were seen to a maximum score of log 2.25 which corresponds with seeing a minimum of 47 letters. Log 1.65 has been reported as a normal value for adults.4 As part of the larger prospective study patients were asked, “Do you often feel sad or depressed,” as a single‐question screen for depression. As a measure of self‐report of independence patients were asked if they were able to prepare all, some or none of their own meals.
Results
Of 225 enrolled patients mean age was 80 years, 141(63%) had age‐related macular degeneration and 70% (157/224) were female. One hundred and forty five (64.7%) reported themselves capable of independently preparing all meals and 80 (35.7%) reported depression.
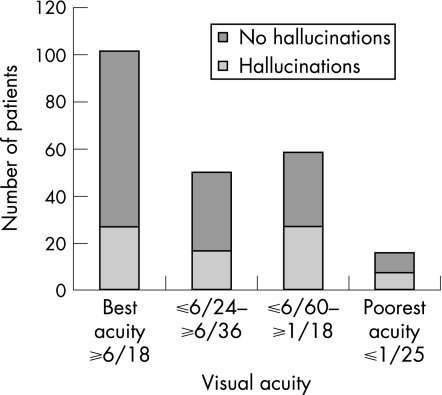
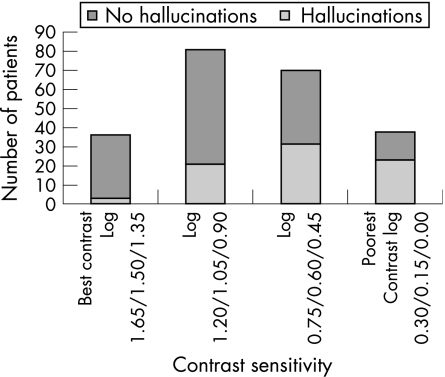
Considering the better seeing eye, 101 (45%) had acuity in the top quartile, acuity better than or equal to 6/18. (table 1) No patients had greater than log 1.65 measured contrast sensitivity and half had poorer than log 0.75 contrast sensitivity and were in the lowest two quartiles (table 1).
Table 1 Distribution of contrast sensitivity and visual acuity in total patient population.
| Contrast Sensitivity log scores | 1.65,1.50,1.35 (best) | 1.20,1.05,0.90 | 0.75,0.60,0.45 | 0.30,0.15,0.00 (poorest) | Total |
|---|---|---|---|---|---|
| Acuity | |||||
| ⩾6/18 (best) | 26 | 48 | 24 | 3 | 101(45%) |
| ⩽6/24‐⩾6/36 | 6 | 24 | 18 | 2 | 50(22%) |
| ⩽6/60‐⩾1/18 | 4 | 9 | 25 | 20 | 58(26%) |
| ⩽1/25 (poorest) | 0 | 0 | 3 | 13 | 16(7%) |
| total | 36(16%) | 70(31%) | 70(31%) | 38(17%) | N = 225 |
Hallucinations were reported by 35% (78/225) of patients. Visual acuity was considered in four quartiles and using the quartile with best function as a reference group and controlling for contrast sensitivity, age, gender, report of depression and independence in multiple logistic regression, measured acuity in each of the poorer three categories was not associated with reported hallucinations (table 2, fig 1). Contrast sensitivity was also considered in quartiles. The three poorer quartiles were strongly associated with the report of hallucinations compared with the top quartile with the best measured contrast sensitivity after controlling for acuity, age, sex, depression and independence (table 2, fig 2).
Table 2 ORs for hallucinations with acuity and contrast sensitivity.
| OR and 95% CI | |
|---|---|
| Acuity ⩽6/24‐⩾6/36 | OR 0.96, CI 0.4, 2.2 |
| Acuity ⩽6/60‐⩾1/18 | OR 0.90, CI 0.4, 2.1 |
| Acuity ⩽1/25 (poorest) | OR 0.30, CI 0.1, 1.3 |
| Contrast sensitivity log 1.20,1.05,0.90 | OR 4.1, CI 1.1, 15.9 |
| Contrast sensitivity log 0.75,0.60,0.45 | OR 10.5 CI 2.6, 42.1 |
| Contrast sensitivity log 0.30,0.15,0.00 (poorest) | OR 28.1, CI 5.6, 140.9 |
Figure 1 Number of patients reporting hallucinations by visual acuity quartiles.
Discussion
In this cohort of patients seeking vision rehabilitation, one third reported recurrent, vivid visual hallucinations on direct questioning. The reported prevalence of hallucinations in visually impaired individuals in Western populations varies widely from 11% to 63% due to differences in populations studied, definition, history taking technique and patients' willingness to disclose the symptom perhaps due to concern that this will imply mental incompetence.1,2,5 Researchers hypothesise that visual hallucinations are due to a lack of true visual input, allowing a cortical release phenomenon similar to phantom limb perception.2 Normally sighted subjects who have bilateral eye patching also report simple or complex hallucinations after an average of one day.6 All of the 78 patients who reported hallucinations in this cohort were aware that the images were not real. Most authors agree that patients have, or develop, insight into the unreality of the images.2 Only a few of our patients were bothered by the hallucinations and other authors also report that only a small subset of patients are disturbed by the hallucinations.2
Our study did not find a significant relationship between poor visual acuity and reported hallucinations. Crane et al also reported a series of 284 patients referred for vision rehabilitation and found no significant relationship between reported hallucinations and measured visual acuity, or the presence and size of a macular scotoma determined by scanning laser ophthalmoscopy.7 Other authors have reported visual hallucinations among patients with normal acuity who have field defects due to glaucoma or hemianopsia.8,9
Contrast sensitivity, the ability to discern shades of grey, is infrequently used as a measure of visual function compared to visual acuity, the latter being the ability to discern detail. Contrast sensitivity has a characteristic curve of contrast threshold over a range of spatial frequency, or size of image. Testing instruments may use many or one size of targets and hence determine the curve, or one point on the curve. The contrast sensitivity curve can be modified both by age and disease states, however, contrast sensitivity for stationary, large images does not change significantly throughout adulthood in healthy eyes.10 The Pelli‐Robson chart is a reliable and quick test of contrast perception of one size of target, a low spatial frequency target, and is valid within a range of illumination settings. Contrast perception of a large target is correlated with difficulty with activities of daily living such as driving or navigating.11,12
Certain geriatric diseases are associated with loss of contrast perception. Patients with Parkinson's disease are recognised to have reduced contrast perception.13 Approximately 25% of patients with untreated Parkinson's disease, who are not demented or psychotic, have been documented to report visual hallucinations. Researchers have noticed an association between poorer contrast sensitivity and hallucinations reported by Parkinson's disease patients.14,15
Figure 2 Number of patients reporting hallucinations by contrast sensitivity quartiles.
Our study found a strong relationship between contrast sensitivity loss and the report of hallucinations in a population seeking vision rehabilitation. An association between contrast sensitivity and hallucinations in patients with vision impairment has not been mentioned previously in the literature to our knowledge. This study has not quantified degree of field loss with report of hallucinations and has not considered contrast perception of other sized targets. Prospectively following patients for the development of hallucinations coincident with loss of contrast sensitivity, or cessation of the symptom of hallucinations with change in visual function would be informative. Given that the geriatric proportion of Western populations is expanding and the fact that vision impairment disproportionately affects the elderly, we can extrapolate that there will be an increasing number of elderly patients who will experience, and may not report, the symptom of visual hallucinations. Discussing this unusual symptom may reassure patients and their families. Recognising a report of visual hallucinations as a symptom of vision impairment, including impaired contrast sensitivity which may be unrecognised, can avoid a misdiagnosis of psychosis, cognitive decline or medication side effect and avoid possible mismanagement. We encourage clinicians seeing elderly patients to ask, “Do you see things which are not there?”
Footnotes
Funding: This study was funded by a CNIB EA Baker Applied Research Grant.
Competing interests: none.
References
- 1.Menon G J. Complex visual hallucinations in the visually impaired. A structured history‐taking approach. Arch Ophthalmol 2005123349–355. [DOI] [PubMed] [Google Scholar]
- 2.Menon G J, Rahman I, Menon S.et al Complex visual hallucinations in the visually impaired. The Charles Bonnet syndrome. Surv Ophthalmol 20034858–72. [DOI] [PubMed] [Google Scholar]
- 3.Jackson M L, Bassett K, Nirmalan P. Charles Bonnet hallucinations; Natural history and risk factors. In: Jones S, Rubin G, Hamlin D, eds. Vision 2005 ‐ Proceedings of the International Congress held between 4 and 7 April 2005 in London, UK
- 4.Elliott D B, Sanderson K, Conkey A. The reliability of the Pelli‐Robson contrast sensitivity chart. Ophthalmic Physiol Opt 19901021–24. [PubMed] [Google Scholar]
- 5.Holroyd S, Rabins P V, Finkelstein D.et al Visual hallucinations in patients from an ophthalmology clinic and medical clinic populations. J Nerv Ment Dis 1994182273–276. [DOI] [PubMed] [Google Scholar]
- 6.Merabet L B, Maguire D, Warde A.et al Visual hallucinations during prolonged blindfolding in sighted subjects. J Neurophthalmol 200424109–113. [DOI] [PubMed] [Google Scholar]
- 7.Crane W, Fletcher D, Schuchard R. Prevalence of photopsias and Charles Bonnet syndrome in a low vision population. Ophthalmol Clin North Am 19947143–149. [Google Scholar]
- 8.Tan C S, Lim V S, Ho D Y.et al Charles Bonnet syndrome in Asian patients in a tertiary ophthalmic centre. Br J Ophthalmol 2004881325–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kolmel H W. Complex visual hallucinations in the hemianopic field. J Neurol Neurosurg Psychiatry 19854829–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Owsley C, Sekuler R, Siemsen D. Contrast sensitivity throughout adulthood. Vision Res 198323689–699. [DOI] [PubMed] [Google Scholar]
- 11.Owsley C, Sloane M. Contrast sensitivity, acuity and the perception of “real‐world” targets. Br J Ophthalmol 198771791–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.West S K, Rubin G S, Broman A T.et al How does visual impairment affect performance on tasks of everyday life? The SEE Project. Salisbury Eye Evaluation. Arch Ophthalmol. 2002 Jun 120774–780. [DOI] [PubMed] [Google Scholar]
- 13.Pierre V, Diederich N J, Raman R.et al Decreased color discrimination and contrast sensitivity in Parkinson's disease. J Neurol Sci 20001727–11. [DOI] [PubMed] [Google Scholar]
- 14.Biousse V, Skibell B C, Wattts R l.et al Ophthalmologic features of Parkinson's disease. Neurology. 2004;Jan 27 62177–180. [DOI] [PubMed] [Google Scholar]
- 15.Diederich N J, Goetz C G, Raman R.et al Poor visual discrimination and visual hallucinations in Parkinson's disease. Clin Neuropharmacol 199821289–295. [PubMed] [Google Scholar]