Abstract
Aims
Hydrogen peroxide (H2O2) is the major oxidant involved in cataract formation. Lens epithelial cells have been suggested to be the first site of oxidative damage. The authors investigated the relationship between H2O2‐induced cytotoxicity and activation of nuclear factor kappa B (NF‐κB) in human lens epithelial (HLE) cells.
Methods
HLE B‐3 cells were stimulated by various concentrations of H2O2 in the presence or absence of pyrrolidine dithiocarbamate (PDTC), a potent inhibitor of NF‐κB. H2O2‐induced cytotoxicity was measured by lactate dehydrogenase cytotoxicity assay. Translocation of NF‐κB was examined by Western blot and immunocytochemistry using anti‐p65 antibody.
Results
H2O2‐induced cytotoxicity increased in a concentration‐dependent manner. PDTC treatment significantly suppressed the cytotoxicity induced by H2O2. After stimulated with H2O2, NF‐κB was found translocated from cytoplasm into the nuclei. PDTC treatment also inhibited the translocation of NF‐κB.
Conclusions
NF‐κB signal pathway may be important in the development of H2O2‐induced damage in HLE cells that is involved in cataractogenesis.
Cataract is a main cause of global blindness and recent data suggest cataract is responsible to 48% of cases of blindness.1 Although it is a multifactorial disease associated with several risk factors, oxidative stress has been suggested as a common underlying mechanism of cataractogenesis. A significant proportion of lenses and aqueous humor taken from cataract patients have elevated hydrogen peroxide (H2O2) levels.2 Because H2O2, at concentrations found in cataract, can cause lens opacification and produces a pattern of oxidation similar to that found in cataract, it is concluded that H2O2 is the major oxidant involved in cataract formation.3 The single layer of lens epithelial cells had been suggested to be the first site of oxidative damage.4 After exposure to oxidative stress, DNA and membrane pump systems of lens epithelial cells are damaged extensively. Then, the cell will die by necrotic and apoptotic mechanisms and loss its cell viability.3
In some cell lines, including the rabbit lens epithelial cell line, H2O2‐mediated oxidative stress has resulted in the activation of nuclear factor kappa B (NF‐κB).5,6 NF‐κB is one of the most ubiquitous transcription factors. It is a multiprotein complex that can activate a great variety of genes involved in early defence reactions of higher organisms. In nonstimulated cells, NF‐κB resides in the cytoplasm in an inactive complex with the inhibitor kappa B (IκB). Pathogenic stimuli cause release of IκB and allow NF‐κB to enter the nucleus, bind to DNA control elements and influence the transcription of specific genes that determine cellular fate and also illicit an immune response to stressed tissue.5,7,8 In different cell types, translocation of NF‐κB could stimulate or inhibit cellular death.9,10 Although in vitro work had demonstrated that H2O2 can activate NF‐κB in rabbit lens epithelial cells and suggested an important role for NF‐κB in modulating oxidative stress in the lens,6 the relationship between H2O2‐induced cellular death and activation of NF‐κB remains unclear.
In order to investigate whether NF‐κB is involved in H2O2‐induced cytotoxicity in human lens epithelial (HLE) cells, activation of NF‐κB was examined by Western blot and immunocytochemistry. The effect of pyrrolidine dithiocarbamate (PDTC), an inhibitor of NF‐κB, on H2O2‐induced cytotoxicity in HLE cells was also investigated to confirm the role of NF‐κB.
Materials and methods
Cell culture and treatments
HLE B‐3 cells obtained from American Type Culture Collections (Manassas, VA, USA) were cultured in Dulbecco's modified essential medium (DMEM; Sigma, St. Louis, MO, USA) supplemented with 20% heat‐inactivated fetal bovine serum (Gibco‐BRL, Grand Island, NY, USA) and antibiotics (100 U/mL penicillin and 100 µg/mL streptomycin) and maintained at 37°C in a humidified incubator containing 5% CO2. The culture medium was replaced by serum‐free DMEM 16 hours before all experiments. 100 μM of PDTC (Sigma, St. Louis, MO, USA) was added into the culture medium 2 hours before H2O2 (Wako Pure Chemical Industries, Osaka, Japan) treatment.
Lactate dehydrogenase (LDH) cytotoxicity assay
Cells were plated at density of 2×104 cells/well in 96‐well plates. After pretreated with PDTC for 2 hours, various concentration of H2O2 was added to the wells for a subsequent 6 hours incubation. The release of LDH in the culture medium was measured by using a cytotoxicity detection kit (LDH) (Roche Diagnostics, Indianapolis Mannheim, Germany) according to manufacturer's instructions. The release of intracellular LDH to the extracellular medium was measured by determining this enzyme activity and was expressed as a percentage of total cellular activity. The absorbance was measured at 492 nm using a plater Reader.
Immunocytochemistry
Cells were seeded onto chamber slides and grown until they were confluent. After treatment with 100 μM of PDTC and 0.5 mM of H2O2 for 1 hour, the slides were washed twice with ice‐cold phosphate buffered saline (PBS), and this was followed by fixation with 100% ethanol for 5 minutes at 4°C. After washing with PBS, the slides were incubated overnight at 4°C with the anti‐p65 antibody (Santa Cruz, c‐20, CA, USA; 1:500). The slides were then washed and incubated in Cy‐3 conjugated goat anti‐rabbit IgG (Jackson ImmunoResearch Laboratories, Inc, West Grove, PA, USA) for 30 minutes at room temperature. Stained slides were examined by laser scanning confocal microscopy (MRC‐1024: Bio‐Rad, Richmond, CA, USA; and LSM 510: Carl Zeiss, Oberkochen, Germany).
Preparation of nuclear extracts and Western blot analysis
Following treatment with 100 μM of PDTC and 0.5 mM of H2O2 for 1 hour, cells were rinsed with ice‐cold PBS/phosphatase inhibitors and nuclear extracts were collected according to the instruction of nuclear extract kit (Active Motif, Carlsbad, CA, USA). Protein concentration was measured with a bicinchoninic acid protein assay kit (Pierce, Rockford, IL, USA). Equal amounts of protein (5 μg per lane) was loaded onto a 10% SDS‐polyacrylamide gels, and transferred to immobilon polyvinylidene difluoride membranes. After blocking, the membranes were incubated overnight at 4°C with the anti‐p65 antibody (Santa Cruz, c‐20, CA, USA; 1:2000), anti‐α‐tubulin antibody (Lab Vision, Westinghouse Drive Fremont, CA, USA; 1:3000). The membranes were then probed with horseradish peroxidase‐conjugated secondary antibody (Amersham Biosciences, Piscataway, NJ, USA; 1:5000) and visualised by chemiluminescence (Amersham Biosciences, Piscataway, NJ, USA).
Statistical analysis
Data were expressed as the mean ± SD. Data were analysed by student t‐test. P<0.05 was considered to be statistically significant.
Results
PDTC attenuates H2O2‐induced cytotoxicity in HLE B‐3 cells
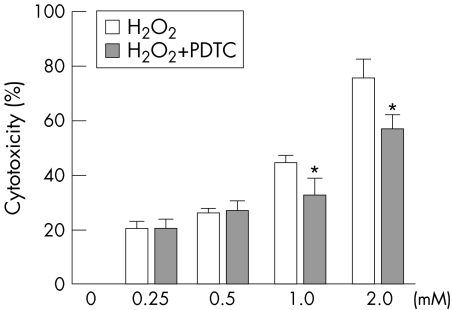
The cytotoxicity increased in a concentration‐dependent manner when exposed to various concentration of H2O2 by LDH cytotoxicity assay. The percentage cytotoxicity was about 20.4% when the cells were treated with 0.25 mM of H2O2 for 6 hours. Higher concentration of H2O2 caused a dramatic increase of cytotoxicity. PDTC treatment showed a significant protection against the toxicity caused by H2O2. After PDTC treatment, the percentage cytotoxicity at 1 mM of H2O2 was lowered from 43.9% to 32.0% and the percentage cytotoxicity at 2 mM of H2O2 was lowered from 75.3% to 56.8%. Cells treated with 100 μM of PDTC alone for 8 hours showed no obvious change in the viability. (fig 1)
Figure 1 Protective effect of PDTC on cultured HLE B‐3 cells against H2O2‐induced cytotoxicity determined by LDH cytotoxicity assay. Cells were pretreated with 100 μM of PDTC for 2 hours before being exposed to different concentrations of H2O2 for 6 hours or were treated only with H2O2 for 6 hours without any pretreatment. *p<0.01 versus the same concentration of H2O2 exposure without pretreatment (mean ± SD, n = 8).
PDTC prevents H2O2‐induced translocation of NF‐κB in HLE B‐3 cells
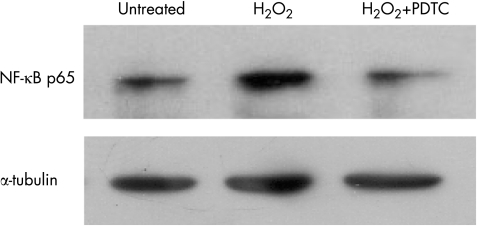
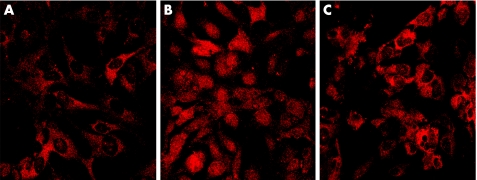
The translocation of NF‐κB was examined by Western bolt and immunocytochemistry. The result of Western bolt showed that considerable NF‐κB signal was detected in the nuclear extract of H2O2‐stimulated HLE B‐3 cells compared with that of unstimulated cells. PDTC treatment significantly lowered the expression of NF‐κB in the nuclear extract. (fig 2) Similarly, the photomicrography of immunocytochemical staining of NF‐κB showed NF‐κB primarily resided in the cytoplasm, not in the nuclei of the unstimulated cells. After stimulated with H2O2 for 1 hour, NF‐κB was found translocated into the nuclei. In the cells treated with PDTC, NF‐κB remained in the cytoplasm, few translocations were found. (fig 3)
Figure 2 Effect of PDTC on H2O2‐induced activation of NF‐κB. HLE B‐3 cells were pretreated with 100 μM of PDTC for 2 hours and then treated with H2O2 at a concentration of 0.5 mM for 1 hour. Nuclear extracts were prepared and then assayed for NF‐κB activation by Western blot. Equal loading of proteins was verified by Western blot analysis of α‐tubulin.
Figure 3 Immunocytochemical analysis of NF‐κB p65 localisation in HLE B‐3 cells after treatment with H2O2 in the absence or presence of PDTC. A: In untreated cells, NF‐κB p65 primarily existed in the cytoplasm, not in the nuclei. B: After stimulated with 0.5 mM of H2O2 for 1 hour, NF‐κB p65 translocated from the cytoplasm into the nuclei. C: In the cells pretreated with 100 μM of PDTC for 2 hours, few NF‐κB p65 translocated into the nucleus.
Discussion
Exposing cells in culture to increasing oxidative stress triggers a range of cellular events. Depending on the cell type, these may include enhanced proliferation or growth arrest, DNA damage, protein and lipid oxidation, apoptosis, and finally necrosis.11 Our results showed H2O2 treatment induced a prominent increase in the cytotoxicity in a concentration‐dependent manner. Lens epithelial cells are vulnerable to the oxidative stress. Exposure to H2O2 can cause cataract and opacification frequently begins in lens epithelial cells. In cell culture, when metabolic systems involved in degrading H2O2 are compromised, epithelial cell death is observed.3 Oxidative stress has been thought as an initiating factor for the development of maturity onset cataract.
There is a report that NF‐κB plays an import role in HLE cellular death after ultraviolet (UV)‐B irradiation and translocation of NF‐κB was observed after UV irradiation.10 Similarly, translocation of NF‐κB after H2O2 stimulation was observed in this study. NF‐κB is crucial for cell survival, cell proliferation and immune responses via expression of its target genes. It is one of the major signal‐transduction pathways that are activated in response to oxidant stress.7 The activation of NF‐κB was based on the detection of its translocation into cell nuclei from its initial location in the cytoplasm, where it exists in an inactive form.10 As our results of immunocytochemistry and Western blot showed, NF‐κB resides in the cytoplasm and there is almost no NF‐κB can be observed in the nuclei of unstimulated HLE cells. After treated with 0.5 mM of H2O2 for 1 hour, a strong translocation of NF‐κB from cytoplasm to nuclei was found in HLE cells. Our results agrees with the results of another study, in which abundance of NF‐κB were observed in the nuclear extract of lens epithelial cells treated with 0.5 mM of H2O2 for 1 hour by electrophoretic mobility shift assays.6
Our data showed that treatment with PDTC markedly blocked the translocation of NF‐κB and resulted in a significant protection against the toxicity caused by H2O2. PDTC has been shown as a potent antioxidant and inhibitor of NF‐κB in vivo and in vitro studies.12 This suggests that the NF‐κB signal pathway may be important in the development of H2O2‐induced damage in HLE cells that is involved in cataractogenesis. However, by which way does H2O2 activate NF‐κB and how NF‐κB affects the gene expression after activation remain unclear. A more thorough understanding of alterations in cellular signaling and transcriptional events as a result of exposure of lens cells to oxidative stress will allow for a more detailed understanding of cataractogenesis and perhaps lead to the development of novel preventive and therapeutic strategies.
Abbreviations
DMEM - Dulbecco's modified essential medium
HLE - human lens epithelial
LDH - Lactate dehydrogenase
PDTC - pyrrolidine dithiocarbamate
Footnotes
Funding: This study is supported in part by a grant for Research on Sensory and Communicative Disorders, Ministry of Health, Labor, and Welfare, Japan, and by Grants‐in‐Aid for Scientific Research, Ministry of Education, Culture, Sports, Science and Technology, Japan.
References
- 1.Foster A, Resnikoff S. The impact of Vision 2020 on global blindness. Eye 2005191133–1135. [DOI] [PubMed] [Google Scholar]
- 2.Spector A, Garner W H. Hydrogen peroxide and human cataract. Exp Eye Res 198133673–681. [DOI] [PubMed] [Google Scholar]
- 3.Spector A. Oxidative stress‐induced cataract: mechanism of action. Faseb J 199591173–1182. [PubMed] [Google Scholar]
- 4.Spector A, Wang G M, Wang R R.et al A brief photochemically induced oxidative insult causes irreversible lens damage and cataract. II. Mechanism of action. Exp Eye Res 199560483–493. [DOI] [PubMed] [Google Scholar]
- 5.Schreck R, Albermann K, Baeuerle P A. Nuclear factor kappa B: an oxidative stress‐responsive transcription factor of eukaryotic cells (a review). Free Radic Res Commun 199217221–237. [DOI] [PubMed] [Google Scholar]
- 6.Dudek E J, Shang F, Taylor A. H(2)O(2)‐mediated oxidative stress activates NF‐kappa B in lens epithelial cells. Free Radic Biol Med 200131651–658. [DOI] [PubMed] [Google Scholar]
- 7.Baeuerle P A, Henkel T. Function and activation of NF‐kappa B in the immune system. Annu Rev Immunol 199412141–179. [DOI] [PubMed] [Google Scholar]
- 8.Boileau T W, Bray T M, Bomser J A. Ultraviolet radiation modulates nuclear factor kappa B activation in human lens epithelial cells. J Biochem Mol Toxicol 200317108–113. [DOI] [PubMed] [Google Scholar]
- 9.Van Antwerp D J, Martin S J, Kafri T.et al Suppression of TNF‐alpha‐induced apoptosis by NF‐kappaB. Science 1996274787–789. [DOI] [PubMed] [Google Scholar]
- 10.Lee D H, Cho K S, Park S G.et al Cellular death mediated by nuclear factor kappa B (NF‐kappaB) translocation in cultured human lens epithelial cells after ultraviolet‐B irradiation. J Cataract Refract Surg 200531614–619. [DOI] [PubMed] [Google Scholar]
- 11.Dypbukt J M, Ankarcrona M, Burkitt M.et al Different prooxidant levels stimulate growth, trigger apoptosis, or produce necrosis of insulin‐secreting RINm5F cells. The role of intracellular polyamines. J Biol Chem 199426930553–30560. [PubMed] [Google Scholar]
- 12.Kitamei H, Iwabuchi K, Namba K.et al Amelioration of experimental autoimmune uveoretinitis (EAU) with an inhibitor of nuclear factor‐kappaB (NF‐kappaB), pyrrolidine dithiocarbamate. J Leukoc Biol 2006791193–1201. [DOI] [PubMed] [Google Scholar]