The enhanced S‐cone syndrome (ESCS), a retinal degenerative disease often associated with NR2E3 mutation,1,2,3,4 is due to increased numbers of S‐cones at the expense of other photoreceptors or miswiring distal to the photoreceptors.5 Adults complain of hemeralopia, and are diagnosed from their unique retinal and electroretinogram (ERG) findings1,2,3,4—characteristic deep clumped pigmentary deposition around the vascular arcades;1,2,3,4 varying degrees of retinoschisis,1,2,3,4 a similar, simplified and delayed ERG waveform response to flashes under photopic and scotopic conditions;2,3 and a delayed 30‐Hz flicker ERG amplitude lower than that of the photopic a‐wave.2,3 Paediatric ESCS and its differing clinical features is the subject of this report.
Case series
Three children were evaluated because of inward eye turn and poor night vision noted since approximately 2 years of age. Retinal examination (fig 1) and cycloplegic refractions were performed 40 min after cyclopentolate 1% drops. NR2E3 was directly sequenced1 from venous blood samples. ERGs, performed under chloral hydrate sedation and according to the recommendations of the International Society for Clinical Electrophysiology of Vision,3 were consistent with ESCS (fig 2). Family history was significant only for case 1.
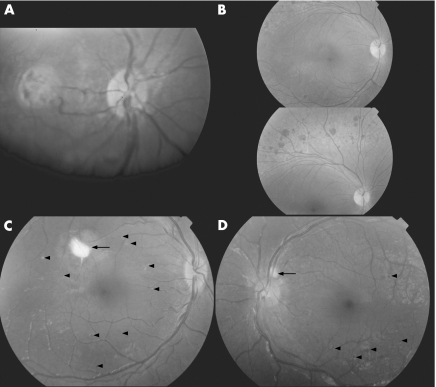
Figure 1 (A, case 1) In both eyes, the retinal pigment epithelium (RPE) had an unhealthy appearance, with scattered subretinal deposits most prominent in the fovea. No pigmentary deposits were seen. (B, mother of case 1) The appearance was classic for enhanced S‐cone syndrome, with deep clumped retinal pigmentary deposits and RPE atrophy outside the vascular arcades of both eyes. Foveal schisis was seen in the left eye (not shown). (C, case 2) A prominent subretinal lesion was seen in a similar location in the posterior pole of both eyes (arrow). In addition, scattered small subretinal deposits and spots of pigments were present (arrowheads). (D, case 3) The RPE had an unhealthy appearance, with scattered small subretinal deposits and subretinal spots of pigment (arrowheads). One larger subretinal deposit was adjacent to the disc in the right eye (arrow).
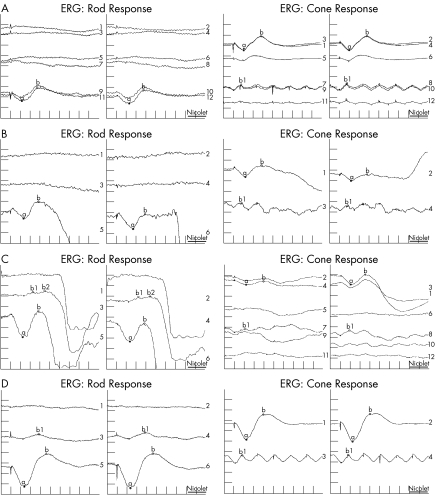
Figure 2 The electroretinograms (ERGs) of (A) case 1, (B) her mother, (C) case 2 and (D) case 3 were diagnostic for enhanced S‐cone syndrome (ESCS)—a similar, simplified and delayed waveform response to flash under photopic and scotopic conditions; and a delayed 30‐Hz flicker amplitude lower than that of the photopic a‐wave. The left tracings of each column are for the left eye and right tracings for the right eye. Stimulus intensities are described in logarithmic (log) units relative to the standard stimulus (1.5–3 Candela seconds/m2 at the surface of the Ganzfeld bowl). For each ERG in the first column (Rod Response), top tracings are for a minimal (0.25 log units) blue (430 nm) stimulus, middle tracings are for a standard (1.0 log unit) red (644 nm) stimulus, and bottom tracings are for a maximal (1.5 log units) white flash. Repeat recordings are seen in (A). In the second column (cone response), for the tracings in (A) and (C), the top tracings are for maximal flash with low background illumination (1 foot Lamberts), the middle tracings for standard flash with high background illumination (30 foot Lamberts), the second to last tracings are for maximal intensity flicker (30 Hz) with low background illumination, and the bottom tracings are for standard intensity flicker with low background illumination. Repeat recordings are seen for these tracings. In the second column for the tracings in (B) and (D), the top tracings are for maximal flash with low background illumination and the bottom tracings are for maximal intensity flicker with low background illumination; no repeats were done.
Case 1 (a 3‐year‐old girl)
The patient's mother had a history of poor night vision for many years. The patient had central/steady/maintained vision in either eye, 18‐prism diopters oesotropia at distance and near, and a cycloplegic refraction of +5.75–1.50×180 OD (right eye), +6.00−1.50×180 OS (left eye). Both eyes had an unhealthy retinal pigment epithelium (RPE) appearance with subfoveal lesions OU (fig 1A). Wearing her full cycloplegic refraction, the patient had no oesotropia. Examination of her mother showed no strabismus, uncorrected visual acuity of 20/60 OD and 20/200 OS, deep clumped pigmentation outside the vascular arcades OU (fig 1B), foveal schisis OS, no significant cycloplegic refraction and an ERG diagnostic for ESCS (fig 2B). Both mother and daughter were homozygous for a previously reported NR2E3 splice mutation (IVS1‐2A→C).1 The father (the mother's cousin) was confirmed as a carrier.
Case 2 (a 5‐year‐old boy)
The patient had a visual acuity of 20/80 OD and 20/40 OS, 20‐prism diopters oesotropia at distance, 25‐prism diopters oesotropia at near, and a cycloplegic refraction of +6.00−2.00×115 OD and +6.00−2.00×75 OS. There were multiple subretinal white deposits and an unhealthy appearance to the RPE (fig 1C). Wearing his full cycloplegic refraction, the patient had no oesotropia at distance and 20/30 vision in either eye. Direct sequencing of NR2E3 was negative. Additional mutational analyses6,7 were not performed.
Case 3 (a 5‐year‐old boy)
The patient, not cooperative for visual acuity testing, had approximately 20‐prism diopters oesotropia at near. Cycloplegic refraction was +7.25 OU. Retinal examination was significant for subretinal white lesions (fig 1D). Wearing his full cycloplegic refraction, the patient had no oesotropia at distance and 20/30 vision OU. NR2E3 sequencing showed homozygosity for a previously reported missense mutation (R311Q, CGG→CAG).1
Discussion
The unique retinal phenotype that typically leads to the diagnosis of ESCS in adults was not observed in the children of this case series. The fact that the affected mother of case 1 exhibited the classic pattern of clumped pigmentation unique to ESCS led us to suspect the diagnosis in her 3‐year‐old daughter and raised our awareness for subsequent similar paediatric patients (cases 2 and 3). Murine models suggest that typical adult retinal phenotype develops over time8. In one previously reported case, the characteristic retinal clumped pigmentation pattern developed between 9 and 11 years of age.4 The authors of that report also believed that significant hyperopia is a recurring feature of ESCS that may be missed without a cycloplegic refraction. Our case series suggests that night blindness, refractive accommodative oesotropia and subretinal lesions are presenting features of ESCS in young children.
Footnotes
This study was approved by the Human Ethics Committee and Institutional Review Board of the King Khaled Eye Specialist Hospital in Riyadh, Saudi Arabia.
Parental/guardian informed consent was obtained for publication of the person's details in this report.
References
- 1.Haider N B, Jacobson S G, Cideciyan A V.et al Mutation of a nuclear receptor gene, NR2E3, causes enhanced S cone syndrome, a disorder of retinal cell fate. Nat Genet 200024127–131. [DOI] [PubMed] [Google Scholar]
- 2.Michaelides M, Holder G E, Moore A T. Inherited retinal dystrophies. In: Taylor D, Hoyt GS, eds. Pediatric ophthalmology and strabismus. 2nd edn. New York: Elsevier Saunders, 2005531–557.
- 3.Fishman G A. The enhanced S‐cone syndrome. In: Fishman GA, Birch DG, Holder GE, Brigell MG, eds. Ophthalmology monograph 2—Electrophysiologic testing in disorders of the retina, optic nerve, and visual pathway. 2nd edn. Singapore: The American Academy of Ophthalmology, 2001120
- 4.Sharon D, Sandberg M A, Caruso R C.et al Shared mutations in NR2E3 in enhanced S‐cone syndrome, Goldmann‐Favre syndrome, and many cases of clumped pigmentary retinal degeneration. Arch Ophthalmol 20031211316–1323. [DOI] [PubMed] [Google Scholar]
- 5.Cepko C. Giving in to the blues. Nat Genet 20002499–100. [DOI] [PubMed] [Google Scholar]
- 6.Wright A F, Reddick A C, Schwartz S B.et al Mutation analysis of NR2E3 and NRL genes in enhanced S Cone syndrome. Hum Mutat 200424439. [DOI] [PubMed] [Google Scholar]
- 7.Nishiguchi K M, Friedman J S, Sandberg M A.et al Recessive NRL mutations in patients with clumped pigmentary retinal degeneration and relative preservation of blue cone function. Proc Natl Acad Sci USA 200410117819–17824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haider N B, Naggert J K, Nishina P M. Excess cone cell proliferation due to lack of a functional NR2E3 causes retinal dysplasia and degeneration in rd7/rd7 mice. Hum Mol Genet 2001101619–1626. [DOI] [PubMed] [Google Scholar]